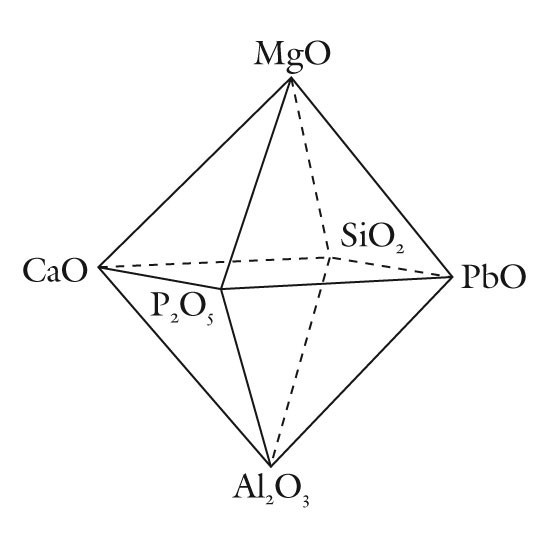
A compositional space diagram for soft-paste porcelains. (Drafted by Randolph Corney; artwork by Nichole Drgan.) Triangular planes from this diagram are used to classify these wares.
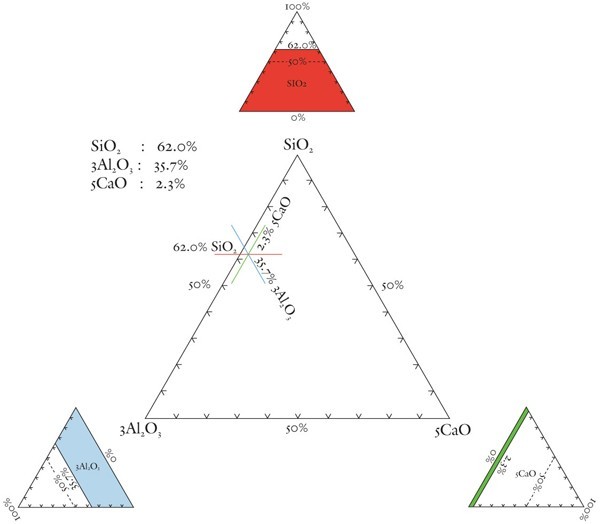
Explanation of the method of plotting analytical data on a triangular diagram. The example chosen is based on paste data for the underfired, aluminous-silicic coffee can illustrated in fig. 10.
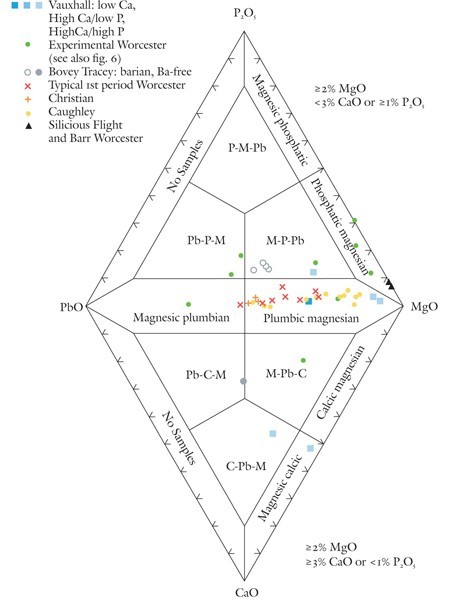
Classification of magnesian porcelains using the P2O5-PbO-CaO-MgO diagram.
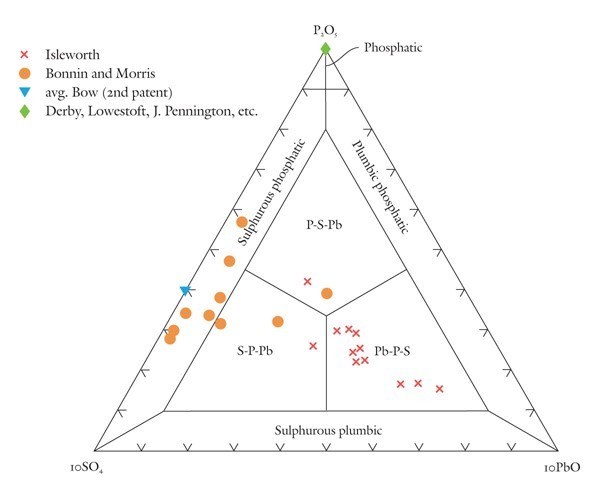
Classification of highly phosphatic porcelains using the P2O5-10SO4-10PbO diagram.
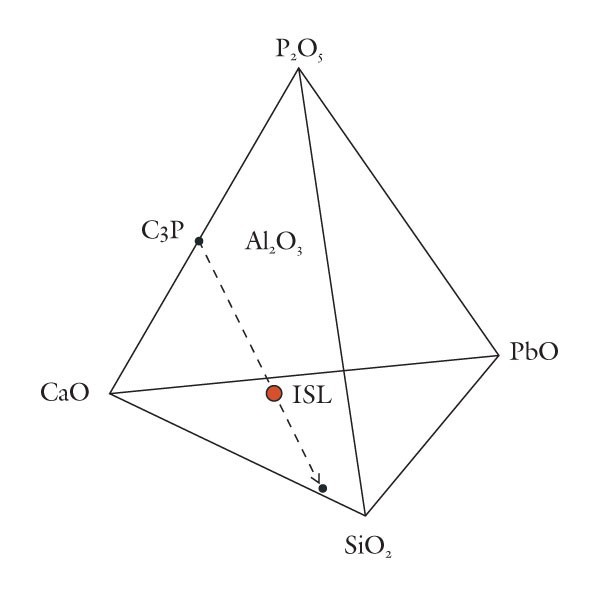
Removal by projection of the phosphate component of variably calcic and plumbian silicious wares.
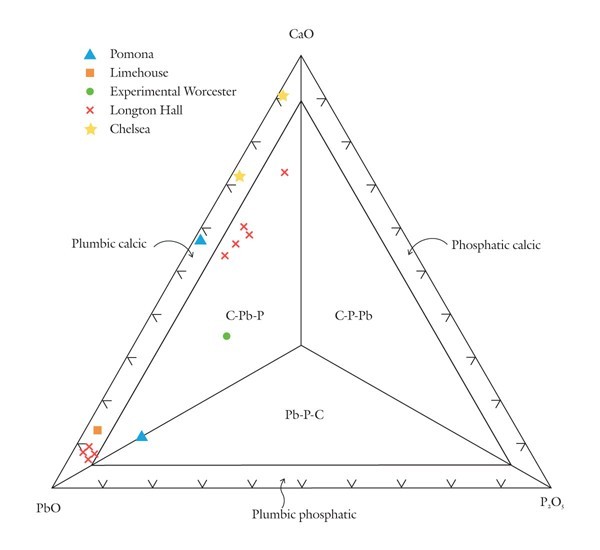
Classification of variably calcic and plumbian silicious wares using the CaO-PbO-P2O5 diagram.
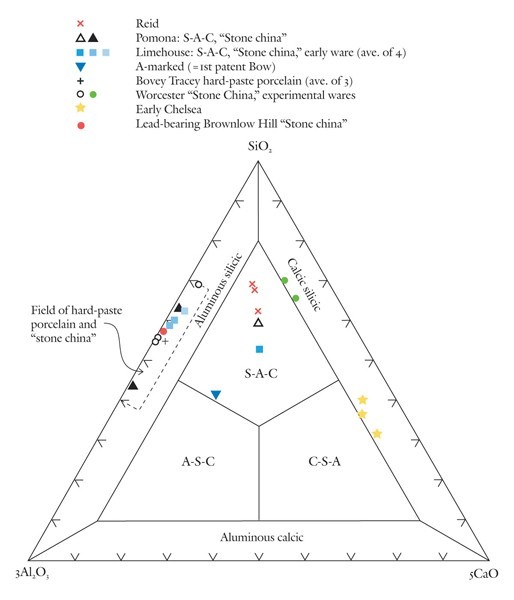
Classification of variably calcic, highly silicious, and aluminous porcelains using the SiO2-3Al2O3-5CaO diagram.
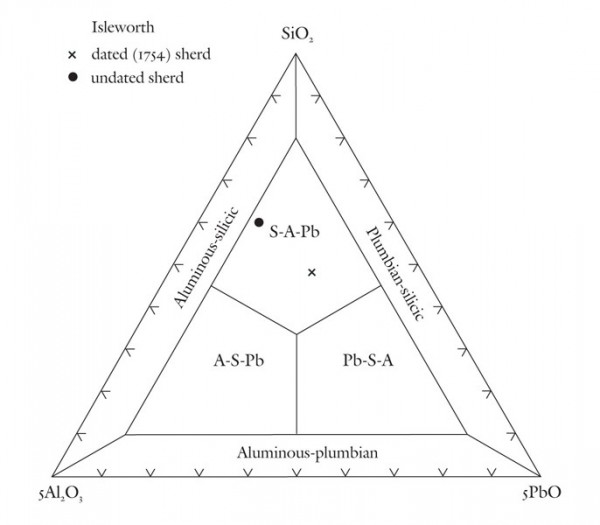
Classification of early, experimental(?) aluminous and plumbian sherds from the Isleworth site using the SiO2-5Al2O3-5PbO diagram.
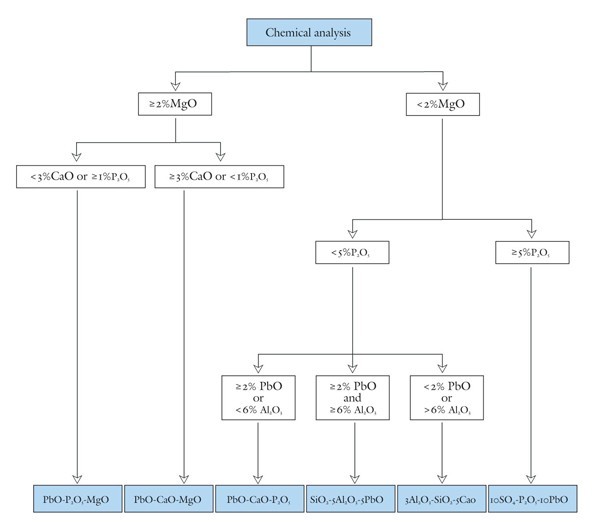
Flowchart governing the selection of appropriate compositional planes for classifying eighteenth-century American and British soft-paste porcelains.

An underfired, aluminous-silicic porcelain coffee can with a lead-rich glaze. H. 2 9/16". (Photo, J. Victor Owen.)
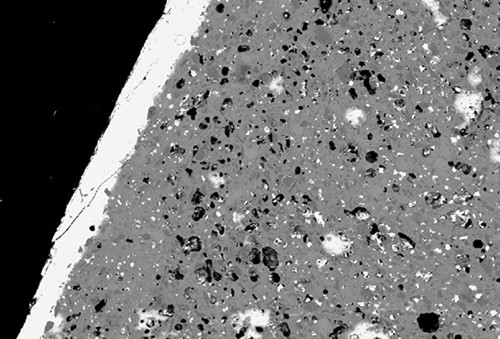
Backscattered-electron image showing the lead-rich glaze (white band) and high porosity (black patches) of the underfired coffee can illustrated in fig. 10. (Image, J. Victor Owen.)
Eighteenth-to early-nineteenth-century soft-paste (or so-called artificial) British porcelains have traditionally been classified using the composition-based scheme—developed by Eccles and Rackham more than eighty years ago—whereby four categories of wares based on inferred paste ingredients were recognized.[1] These porcelain categories are “glassy,” “bone” (bone ash), “soapstone,” and “hybrid” or “modern bone.” Compositionally, the first three of these wares are rich in lead (Pb), phosphorus (P), and magnesium (Mg), respectively. Like bone-ash porcelain, bone china is also phosphatic, but its paste includes materials used in the preparation of hard-paste (“true,” or Chinese-type) porcelain. Some of the key ingredients thought to have been used in the manufacture of these wares are listed in Table 1.[2] In some instances, factory records confirm the identity of these paste ingredients; in others, they must be inferred based on the ware’s composition.
Although they require chemical analysis or X-ray diffraction traces to identify their key constituents, these categories have served porcelain aficionados well. However, recent analysis of American and British soft-paste porcelains has revealed a broader range of wares than those described by Eccles and Rackham, including a number of newly recognized compositional intermediaries (hybrids) between the original end-member groupings and altogether different categories of wares that defy being readily assigned to any of the traditional categories. Some hybrid wares apart from bone china have been known for decades. They include lead-bearing magnesian porcelain (a hybrid between glassy and soapstone) made primarily at the Worcester, Christian, Caughley, and Vauxhall factories (Table 2). Ironically, it is only recently that essentially lead-free soapstone porcelain has been recognized. It was found within a small group of diverse, experimental wares from the earliest days (early 1750s) of the Worcester manufactory and is also represented by some relatively silicious (~82% versus ~75–80% silica [SiO2]) but less aluminous (~8% versus ~15–20% alumina [Al2O3]) porcelain produced during the factory’s Flight and Barr period (1792–1804).[3]
Recently recognized hybrids include lead-bearing bone-ash porcelain (e.g., Bonnin and Morris, Isleworth) and mildly phosphatic soapstone porcelain (early 1750s experimental Worcester).[4] An altogether new class of ware—represented by a type of porcelain with high concentrations of SiO2, Al2O3, and CaO (calcium oxide, or lime)—apparently corresponds to first-patent Bow (i.e., “A-marked” porcelain) and compositionally kindred wares recovered from the sites of the Pomona, Limehouse, and Reid factory sites.[5] This type of ware has been called “clay-rich soft-paste” porcelain.[6] Since analysis of A-marked porcelain indicates derivation from a paste containing significant amounts of both glass and clay components, it is unclear whether it should be described as glassy or clay-rich.[7] Consequently, because the chemical composition of these porcelains is SiO2-Al2O3-CaO rich, they have been called “S-A-C” wares.[8] Rather than relying on inferred paste ingredients to name them, this latter approach emphasizes their chemical composition. This is a much more objective strategy, as it eliminates the need to surmise or guess the nature of the original paste ingredients.
Owing to the nomenclature problems presented by these diverse and novel wares, and the difficulties inherent in inferring the nature of their original paste ingredients, a reconsideration of the classification of early soft-paste British porcelain is warranted. The merits of alternative nomenclature schemes will be considered after salient features of the traditional porcelain groupings are reviewed.
Traditional Soft-Paste Porcelain Groupings
Glassy porcelains. The term glassy (or frit) porcelain is problematic. Indeed, it is surprising that the term has ever been used to describe any of the soft-paste porcelains, because these wares are much less vitrified and therefore inherently less vitreous (glassy) than hard-paste porcelain. Rather, this term appears to have been used to describe porcelains thought to have been made from paste recipes containing glass. That early porcelain manufacturers would have included glass in their ceramic pastes is hardly surprising, since it is in its translucency and whiteness that porcelain diVers from earthenware. The firing at high temperature of glass- and clay-rich mixtures is a logical approach to achieving these attributes. In Britain the glass used for this purpose was generally (but not exclusively, viz. A-marked porcelain) rich in lead. In that country, therefore, glassy porcelain has come to be linked to wares containing this component.
These so-called glassy wares are also commonly referred to as “frit” porcelains, because of the inclusion of a specially prepared glass frit in the pastes from which these wares were made.[9] However, many other types of ware are also produced in this way (Table 1), although the frit was not necessarily lead rich. For example, factory records demonstrate that phosphatic Nantgarw porcelain (South Wales, ca. 1813–1820) was made from a frit-bearing paste. This frit contained 13 parts bone ash, 7 parts Lynn sand (presumably from Kings Lynn, Norfold County, England), and 1 part potash (K carbonate).[10] After being melted, quenched, and powdered (fritted), it was mixed with clay in the ratio 2:1. Although the Nantgarw paste contained frit, those wares are never called frit porcelain, probably because they are highly phosphatic and lead free.
Blending descriptive, interpretative, and compositional connotations of the word glassy is obviously unacceptable. Moreover, it is not necessarily the case that the lead in some “glassy” wares originated in lead-rich flint (colorless) glass or smalt (blue potash-lead glass). It might well have been—smalt has been recovered from both the Caughley and the Bonnin and Morris factory sites—but this cannot readily be determined after the fact. It would be difficult to disprove the possibility that in some instances compounds such as litharge (PbO), red lead (Pb3O4), or cerussite (PbCO3) were used instead of potash-lead glass. For example, it has been suggested that cerussite was used in “glassy” Derby recipes.[11] However, such is the bias in favor of a glass source for this component, and importance placed on this ingredient in naming these wares, that even comparatively lead-poor (~2% PbO) porcelains such as some Chelsea wares have been included in the “glassy” grouping despite the fact that other wares with much higher lead contents are given other (admittedly hybrid) designations. The latter, of course, include most of the so-called soapstone porcelains.
The terms glassy and frit are therefore inappropriate on three counts: (1) the wares so named are not as vitreous as true porcelain; (2) some lead-free wares used either a specially prepared glass frit or a preexisting soda-lime (or other lead-free) glass in their manufacture; and (3) the source of the lead, as is generally present in British “glassy” wares, might not have been a preexisting lead-rich glass. This usage of the terms glassy and frit should be abandoned.
Soapstone porcelain. There is no doubt that soapstone—i.e., steatite, sensu lato—was used in the preparation of some early British porcelains. This innovation dates at least as far back as the late 1740s, when Benjamin Lund’s Bristol factory commenced operation. The soapstone lent plasticity to ceramic pastes, partly replacing the clay that was generally used for this purpose. It originated in quarries on the Lizard peninsula in Cornwall, and records of licenses to mine this rock have been widely reported in the literature. Evidence for the use of soapstone is provided by the magnesian character of these wares and by the presence of Mg- and/or Ca-Mg silicate minerals (i.e., enstatite and diopside, respectively). However, other commonly used paste ingredients can contain magnesium. Limestone (CaCO3), for example, was used in some ceramic pastes, and this carbonate rock is commonly dolomitized (i.e., enriched in CaMgCO3 components). This appears to be the source of magnesium in some soda-lime glass.[12] In addition, plant ashes—a widely used source of alkali fluxes in early porcelains—can contain as much magnesium as they do sodium and potassium; some types of clay also contain this component.[13]
Of course, the bulk of magnesium in porcelain made by factories known to have held appropriate quarrying licenses most assuredly would have originated in soapstone. This, however, sheds little light on the nature of the material itself, because “soapstone” has a different meaning depending on whether it is used to refer to a mineral or to a rock. If used as a mineral name, it probably refers to steatite (massive talc, a hydrated Mg silicate). If used as a rock name, its meaning is broader, and generally refers to a metamorphic rock “composed essentially of talc with varying amounts of micas, chlorite, amphibole, pyroxenes, etc.”[14]
In the case of soapstone from the Lizard peninsula, associated minerals can also include serpentine (another hydrated Mg silicate), notably lizardite, which is named after this locality. Indeed, soapstone samples from the Worcester factory’s quarry at Gew Graze revealed only traces of talc, making it difficult to reconstruct exactly what type of material—loosely described at the time as “soapy rock”—was added to so-called soapstone porcelain pastes.[15]
Regrettably, the mineralogy of early magnesian porcelain only constrains the nature of this material broadly, because after firing, different hydrated Mg silicates (e.g., talc, serpentine, or Mg-rich amphibole) can break down into the same minerals found in these areas (e.g., enstatite). Owing to these uncertainties, the vague genetic modifier soapstoneshould be abandoned.
Bone-ash porcelain. The addition of bone ash to ceramic pastes was the major innovation made by early British porcelain manufacturers. Over time, some of the ingredients used in bone-ash wares were combined with true porcelain paste components, resulting in bone china, which remains an industry standard in the United Kingdom.
Bone-ash porcelains have phosphatic compositions. Bone ash is virtually the only source of this component in these wares, since other phosphate minerals are not known to have been deliberately added to porcelain pastes, and phosphate minerals generally occur in extremely low concentrations in quartz-rich sand (and not at all in flint, another source of silica in some wares). The only contentious issue concerns the temperature at which the bone was calcined (fired) prior to its use in ceramic pastes. As bone is high-fired (to temperatures of about 1,000° C), it progressively dehydrates and loses some of its lime.[16] If low-fired, the bone ash might account for all of the lime in the analyzed ware under consideration, so that the addition of limestone would not be required should the porcelain have a higher CaO/P2O5 ratio than high-fired bone ash. Uncertainties in the character of the bone ash do not justify abandoning the use of the term when describing phosphatic porcelains. However, in keeping with problems associated with other terms (glassy, soapstone) used to describe traditional end-member porcelain types, and, as is discussed below, the recognition of small amounts of relict bone ash in some of them (e.g., Longton Hall) and of lead in some “bone-ash” porcelains (e.g., Bonnin and Morris, Isleworth), an alternative nomenclature for these wares should be considered.
Bone china. Bone china was developed in the 1790s by Josiah Spode. It is a hybrid of key ingredients used in traditional bone-ash and true porcelains, the latter being made from pastes containing china clay (kaolinite) and china stone (hydrothermally altered granite). Its name is too entrenched to be replaced now, since this ware has been an industry standard in the U.K. for two centuries. Nevertheless, caution should be exercised when applying the term to early phosphatic wares because, compositionally, bone china can resemble bone-ash porcelains that did not include altered granite in their pastes. They differ insofar as historical bone china generally has higher alumina (typically 12–15% versus 6–12% Al2O3) and potash (~1.5–2.5% versus <1.5% K2O) contents than its phosphatic predecessor.[17] Furthermore, at least until the discovery in the late 1760s of domestic supplies of kaolinite, bone-ash porcelains were made using ball clay, which, even after washing, tended to retain some impurities such as FeTi oxide minerals. Thus, they typically contain between 0.2% and 0.5% TiO2, whereas this component usually comprises <0.1% of bone china. The character of some of the other paste ingredients (notably the source of K2O) nonetheless remains problematic.
A New Classification Scheme for Soft-Paste Porcelains
Ceramics are essentially synthetic rocks. In the case of porcelain, they have been sufficiently high-fired for partial melting to have occurred. Given the rapid cool-down period (a day or two) in the kiln, the melt phase is generally preserved as a glass that might contain micron-scale crystallites.[18] This, together with their very fine grain size, begs comparison between porcelains and volcanic rocks, at least from the perspective of classifying them.
Volcanic rocks, of course, are derived from the quick cooling of magma that has been extruded onto Earth’s surface. They are classified according to texture, mineralogy, and chemical composition, or some combination thereof. Some of the minerals found in igneous rocks, including volcanics, are incompatible with others, so they never form in equilibrium with one another. This has led to separate classification schemes based on the more common of these mineral incompatibilities (i.e., between quartz and feldspar versus feldspathoids). For light-colored igneous rocks rich in quartz (or feldspathoids) and feldspars, this has led to the diagrammatic portrayal of mineralogy-based schemes, with quartz at the top, feldspathoids at the bottom, and two types of feldspar (K and plagioclase) on either side resulting in a diamond-shaped plot.[19] This approach requires that the volume percentage of each of these minerals in the rock (i.e., its modal composition), recalculated to 100% exclusive of others, be determined. With practice, mineral proportions in coarse-grained rocks can be estimated visually, so these classification schemes are useful in field-based work (i.e., geological mapping). It can be challenging and time-consuming, however, to accurately measure the modal composition of rocks, so petrologists have devised a scheme to manipulate bulk chemical compositional data in such a way that a hypothetical mineralogy can be calculated for individual samples. This “normative” mineralogy attempts to mimic the natural sequence in which different minerals crystallize from magma as it slowly cools. Rules governing this petrological accounting scheme were first established a century ago.[20] The advantage of this approach is that the (hypothetical) modal composition of analyzed samples can be calculated rather than measured. Furthermore, it takes into account any glassy material that might be present. Indeed, volcanic rocks, many of which contain a glassy phase, are routinely classified on the basis of their normative mineralogy.[21]Alternatively, bulk-rock compositional data can be used to classify igneous rocks directly, without any further manipulation. This can be done either to name individual rock samples or to characterize suites of genetically related (comagmatic) rocks.[22]
Based on petrological precedents, we are thus presented with three possible approaches by which a new, compositionally based classification scheme for early porcelain can be constructed. A mineralogy-based scheme is impractical owing to difficulties in acquiring modal data and the fact that the mineralogy of porcelain is partly governed by firing history. A normative mineral-based scheme is more practical once bulk compositional data are available for the body of porcelain samples. Using these data, the normative mineralogy of individual samples can be determined using existing petrological software. In the case of lead-bearing wares, however, the software would have to be modified to allow for the formation of a lead silicate (e.g., 3PbO*2SiO2 or another plumbian phase[s]) late in the “crystallization” sequence reconstructed by the program. The determination of mineral norms, however, seems to be an unnecessary additional step once bulk, major element compositional data have been acquired. These data alone are sufficient to classify early porcelain.
The composition of most soft-paste porcelain is dominated by some combination of SiO2-Al2O3-MgO-CaO-PbO-P2O5. Therefore, a graphical classification scheme can be based on planes delineating the planes within or on the sides of a three-dimensional figure based on these six components (fig. 1). Small amounts of alkalis are invariably present, so these components (Na2O and K2O) can be disregarded for classification purposes since their rather limited range in concentrations fails to distinguish between many types of soft-paste porcelains. Some wares contain novel components, such as barium, but these seem to be excessively rare and so can be omitted from general classification schemes. As will be seen, small amounts of sulphate (or lead) can serve to distinguish between different types of common phosphatic wares, so this component may potentially be used to help classify this particular category of porcelain. It certainly serves as a signature component that helps to identify the products of some factories such as second-patent Bow porcelain (1748; Table 2). This highlights an ancillary aspect of any proposed classification scheme: not only should such a scheme be readily applied but it should be useful. From the perspective of museum curators, ceramic historians, and collectors, the most useful nomenclature scheme would link a particular category of ware to a restricted group of factories. In this regard, the most abundant components (such as silica) need not necessarily be included in a ceramic classification scheme because minor components alone may be diagnostic.
One obvious problem that must be addressed concerns limitations on the graphical portrayal of any classification scheme being proposed. Complex media such as soft-paste porcelain can contain half a dozen or more major and minor components and not all can be included on a two-dimensional plot. Unless some are added together, we are confined to displaying three, or possibly four, components on a two-dimensional surface. Four components can be used if a three-dimensional image (e.g., a tetrahedron) is depicted, but this creates problems in plotting and visualizing points within it. In some instances, this problem can be overcome by projecting points that plot in the interior of the tetrahedron onto one of its faces. This reduces by one (from four to three) the number of components that needs to be displayed. An example of this approach is considered below. In the meantime, however, six compositional planes (P2O5-PbO-MgO, CaO-PbO-MgO, P2O5-SO4-PbO, CaO-PbO-P2O5, SiO2-Al2O3-CaO, and SiO2-Al2O3-PbO) will be considered. They serve to describe the compositional heterogeneity of early soft-paste American and British porcelains. Their application to the classification of these wares is described in the following sections. Those unfamiliar with plotting data on triangular diagrams should be aware that to do so, the analytical data for the components of interest which are shown on the diagram’s apices must first be recalculated as percentages. An example is shown in figure 2.
The P2O5 -PbO-MgO and CaO-PbO-MgO Planes
So-called soapstone porcelains are rich in magnesia (about 4–20% MgO) and almost invariably contain substantial amounts of lead. Although soapstone largely replaced clay in these wares, they still contain a few percent alumina. Pure talc contains none of this component. There are two main variants of these wares. Although most contain small concentrations of lime (generally < 3% CaO), some are enriched (typically ~8% CaO) in this component. These differences are reflected in their mineralogy. Lime-poor specimens contain enstatite, an orthopyroxene; lime-rich ones can also contain diopside, a clinopyroxene. To date, lime-rich magnesian porcelains are known to have been produced commercially only at the Vauxhall factory, which also manufactured low-Ca plumbic magnesian wares like the Worcester, Caughley, and Christian (Liverpool) factories. Diopside-bearing magnesian porcelain sherds, however, were also recovered from the Bovey Tracey factory site in Devon, but no intact examples of these wares have been conclusively identified. Some of these sherds have barian compositions (~3% BaO).[23]
The lime in these wares likely originated in limestone or possibly, in some instances, in soda-lime glass, although this remains to be shown. However, some Vauxhall and experimental early Worcester porcelain, as well as magnesian Bovey Tracey wares, contain relict bone ash, another source of this component. In order to help distinguish between the different sources of lime in these magnesian porcelains, as well as highlight their variable Mg/Pb ratios, use is made of a diamond-shaped plot with PbO, P2O5, MgO, and CaO on its apices. Compositional criteria for selecting which part of the diagram (top or bottom) is appropriate for magnesian porcelains are indicated in figure 3. These criteria will likely exclude the compositions of any porcelains from plotting in the low-Mg fields hugging the P2O5-PbO and PbO-CaO joins, so these areas are not labeled. These include some of the highly silicious, mildly phosphatic but Mg-poor experimental Worcester porcelain associated with the more magnesian sherds found at the factory site.[24] Their compositions can be plotted on other classification diagrams, as is discussed below.
Seven fields are labeled on the P2O5-PbO-MgO (top) part of the diagram. Porcelains with compositions suitable for making use of this diagram (see screening criteria in fig. 3) include commercially successful magnesian wares as well some of the important experimental pastes produced at Worcester during its nascent years (ca. 1751 to the mid-1750s).
Typical first-period (i.e., Dr. Wall era) Worcester porcelain produced between the mid-1750s and 1770s and one sample of an early 1750s experimental ware have compositions that plot near the upper half (right side) of the PbO-MgO join. So too do the compositions of Caughley, Christian, and some Vauxhall porcelains. These wares are referred to here as plumbic magnesian porcelain. The composition of one sample of experimental Worcester plots near the lower half of the PbO-MgO join, in the magnesic plumbian porcelain field. Three other experimental porcelains from the Worcester site plot near the upper half of the MgO-P2O5 join, in the phosphatic magnesian field. These are low-lead variants, moderately phosphatic wares that evolved into the more traditional plumbic magnesian porcelains that the Worcester factory produced from the mid-1750s onward.[25] Two highly silicious, lead-free Worcester porcelain samples from the Flight and Barr period (1792–1804) also have compositions that plot in this field.
Some magnesian wares have compositions that plot in the interior of the P2O5-PbO-MgO diagram; this area is divided into three trapezoidal fields. Most of these samples plot in what is abbreviated here as the M-P-Pb field. They represent experimental Worcester and barium-bearing Bovey Tracey magnesian porcelains that contain substantial amounts of both phosphate and lead. One sample of high-Ca Vauxhall porcelain also plots in this field. In contrast to its counterparts that plot in the lower half of figure 3, much of the lime in this Vauxhall sample originates in bone ash, relicts of which are preserved in this analyzed specimen.
The lower part of figure 3 is reserved for relatively calcic but phosphate-poor magnesian wares. Single samples of experimental Worcester, Ba-free Bovey Tracey, and high-Ca/low-P Vauxhall porcelain plot well into the interior of the CaO-PbO-MgO diagram. Each is confined to different, labeled fields. Another high-Ca/low-P Vauxhall sample is depleted in lead and therefore plots in the magnesic calcic field on this diagram.
The P2O5-PbO-CaO-MgO diagram allows a number of the historically important, variably magnesian, experimental wares produced by some eighteenth- to early-nineteenth-century factories to be systematically named. The scattering of points representing this material reflects the variety of porcelain pastes that these manufacturers tinkered with before stumbling on what became a more successful type of ware that is more properly described as “plumbic magnesian” than “soapstone” porcelain.
The P2O5-SO4-PbO Plane
Traditional phosphatic porcelains were made of pastes containing approximately 20–35 wt% bone ash. However, some of these wares contain small amounts of sulphate that likely originated in the use of gypsum. Others can contain minor amounts of lead derived from potassic-lead glass or from the addition of red lead or another lead compound to the bone-ash paste. Although the concentrations of sulphate and lead are minor compared with some other components, they do help to distinguish the wares produced by different factories. As such, they are useful components, once scaled, to exploit in a composition-based nomenclature scheme for phosphatic porcelains. In the present case, both components are scaled tenfold. Because of this scaling, fields hugging the edges of the diagram below the phosphatic field at its apex (fig. 4) are not subdivided.
This diagram shows that many phosphatic wares have compositions that plot on the P2O5 apex of the diagram. These are, of course, free of sulphate and lead. They include some of the products of the Derby, Lowestoft, James Pennington, and Coalport factories.[26] In contrast, the Bonnin and Morris factory produced a sulphate- and lead-bearing phosphatic porcelain with compositions that plot mostly in the phosphatic sulphurous field but also extend into the interior of the P2O5-SO4-PbO diagram.[27] Second-patent Bow porcelain produced lead-free, sulphate-bearing wares with compositions that plot in the lower half of the sulphurous phosphatic field. Most Isleworth porcelain plots well in the interior of the diagram, in the Pb-P-S field. As is discussed below, however, two sherds of an apparently earlier-produced type of porcelain from the Isleworth site have silicious and aluminous compositions and must be classified using another diagram.
The CaO-PbO-P2O5 , SiO2-Al2O3-CaO, and SiO2-Al2O3-PbO Planes
The term glassy traditionally has been used to describe variably lead-bearing, lime-rich Chelsea and Longton Hall porcelains.[28] Their elevated lime (~8–20% CaO) and silica (~62–77% SiO2) and comparatively low alumina (~3–4% Al2O3) contents ensured that a calcium silicate mineral, wollastonite (CaSiO3), rather than calcium feldspar (anorthite, CaAl2Si2O8) formed and was preserved during the firing of these wares.
Analysis of wasters from the Reid (Brownlow Hill), Pomona, and Limehouse factory sites shows that there is a wide range of compositional variants of what at one time might have been called glassy porcelains. None, however, contains discrete crystals of wollastonite, although wollastonite occurs as symplectic (“wormy”) intergrowths with diopside and silica polymorphs in Limehouse porcelain.[29] The apparent lack of this mineral in the other wares is likely due to their relatively low lime content (~4–7% CaO).
Compositionally, non-phosphatic Reid porcelain is particularly distinctive because it is highly silicious (82–85% SiO2). It contains 6–8% alumina and 3–5% lime. Collectively, silica, alumina, and lime constitute 92–95% of the bulk composition of this ware, which consequently has been referred to by the acronym “S-A-C” porcelain.[30] This is an altogether new category of wares that does not correspond to traditional ones. Variants from the Pomona and Limehouse sites diVer in their silica and lime contents, but all contain small amounts of lead, the signature ingredient of traditional glassy porcelains. First-patent Bow porcelain, as apparently represented by A-marked wares, is much more aluminous (and depleted in silica) but can also contain small amounts of lead and is still dominated by the three components that characterize these diverse S-A-C wares.
Classification of S-A-C and the traditional glassy porcelains is challenging because of their compositional variation. Even those in the latter category can diVer markedly from one another in terms of their basic paste ingredients. Some Longton Hall porcelain, for example, contains relict bone ash that accounts for significant amounts of phosphate (~2–3% P2O5). This component is virtually absent in glassy Chelsea porcelain. Furthermore, unlike that of Longton Hall, the lead content of early Chelsea porcelain can vary widely.
Based on these observations it seems appropriate to use different compositional planes to subdivide and distinguish the broad grouping of S-A-C–type and compositionally overlapping glassy porcelains. It would, of course, be advantageous to try to bridge these two groupings by linking some of these components. This could be achieved by selecting four appropriate components that represent the apices of a tetrahedron. Then, porcelain compositions that plot in its interior can be projected onto one of the tetrahedron’s faces. This plane would then serve to classify wares whose compositions are represented by the quaternary (simplified, four-component) system. For example, most Longton Hall and late Isleworth porcelains are dominated by silica, lime, lead, and phosphate, although the latter ware has much higher P2O5/PbO ratios than the former. Their bulk compositions can be projected onto the SiO2-CaO-PbO face of the SiO2-CaO-PbO-P2O5 by projecting from a point corresponding to the composition of high-fired bone ash [3CaO * P2O5 ]. This procedure is shown graphically in figure 5. It can be done mathematically, but the measured oxide data must first be divided through by their respective molecular weights (e.g., silica = 60.09). The CaO content of the projected point is determined by reducing the measured amount of this component by the lime removed with the phosphate residing in the relict bone ash (i.e., = CaO - 3P2O5, expressed as molecular proportions). However, because of the high molecular weight of PbO (= 223.2) and comparatively low concentrations of this component in these wares, the projected points lie close to the CaO-SiO2 join (fig. 5). This limits the practicality of this approach for classifying porcelain. Instead, the high silica content of these wares is disregarded and use is made of a CaO-PbO-P2O5 diagram for classifying traditional “glassy” wares (fig. 6). The broad range of S-A-C porcelains will, appropriately enough, be subdivided according to their silica, alumina, and lime content (fig. 7). These diagrams can thus be used to name Mg- and P-poor, variably lead-bearing, and lime-rich porcelains. Figure 6 is intended for relatively lead-rich (>2% PbO) or low-alumina (<6% Al2O3) examples of these wares, whereas figure 7 can be used for lead-poor specimens (<2% PbO) or aluminous (>6% Al2O3) wares. Figure8 is suitable for low-Mg, low-P (and low-Ca) wares with substantial lead (>2% PbO) and alumina (>6% Al2O3) contents. To date, only two analyzed artifacts known to the author have compositions appropriate for classification by this latter diagram. One of these samples, a lead-bearing, highly silicious and aluminous sherd from the Isleworth pottery site dated 1754 (possibly 1756) is a novel hybrid ware that predates the better-known phosphatic porcelain apparently produced by this London potworks after about 1760.[31] That piece and one other equally silicious (but less lead-rich) undated sherd from the Isleworth site are thus far unique among early British wares. Both samples are appropriately referred to as S-A-Pb porcelain (fig. 8).[32]
The CaO-PbO-P2O5 diagram (see fig. 6) is divided into six fields. The compositions of lead-bearing Chelsea samples plot in the plumbic-calcic porcelain field. Some Pomona, Longton Hall, and Limehouse porcelains also consist of this type of ware, but they are comparatively rich in lead. Relatively phosphatic Longton Hall porcelain and one experimental Worcester sample plot in the interior of the diagram, in the C-Pb-P porcelain field. A Pomona sherd plots on the boundary between the C-P-Pb and Pb-P-C fields.
The SiO2-Al2O3-CaO plot is scaled here to spread paste analyses to more aluminous and calcic compositions in the interior of the diagram (fig. 7). This scaling of alumina (x3) and lime (x5) ensures that the compositions of non-phosphatic Reid porcelain plot in the S-A-C field. So, too, do selected A-marked, Limehouse, and Pomona samples. Low-lead Chelsea and some experimental Worcester porcelain have compositions that plot in the calcic-silicic porcelain field. Because of their low lime content, hard-paste porcelain and stone china compositions plot in the aluminous-silicic porcelain field, a reasonable designation for these wares. The compositions of an experimental Worcester sherd, a lead-bearing (1.8% PbO) Brownlow Hill (Reid) sherd, and early alkali-lime glazed Limehouse porcelain also plot in this field.[33] The convergence of some soft-paste wares into that part of the aluminous-silicic field dominated by hard-paste porcelain and stone china (see dashed box in fig. 7) highlights the compositional similarity of what previously have been considered to be separate categories of wares. This is but one of several topics worthy of further discussion.
Discussion
Classification schemes for petrological materials such as fine ceramics should be based on objective criteria that do not require assumptions about the identity or initial character of the medium prior to firing (i.e., its original paste ingredients). The classification scheme presented here is based solely on the bulk chemical composition of the body of analyzed soft-paste porcelains. In this regard, its use requires none of the speculation that has for decades plagued the study of early porcelain. Although more detailed, it mirrors the nomenclature of glass, which is based on the relative abundance of calcium, sodium, potassium, lead, and boron (resulting in categories such as soda-lime, potash, potash-lead, and borosilicate glasses).[34]
Once a bulk chemical analysis is available for the body of a soft-paste porcelain object of interest, its application simply requires following a flowchart (fig. 9) to select the appropriate diagram and then plotting the appropriate components on it. The screening criteria are based on the concentrations of MgO, P2O5, CaO, and PbO. A fundamental division is based on bulk magnesia contents. An MgO value exceeding 2 wt.% was chosen to distinguish magnesian wares because some detrital clays (e.g., pelagic clay) can contain upwards of 4% MgO (although some specific clay species [smectites, vermiculite] can contain five times this much magnesia), and soft-paste porcelains at most contain about 50 wt.% clay components, and these aluminous soft-paste wares tend to be phosphatic in composition.[35] Although small amounts of the magnesia in these Al-rich, Mg-poor porcelains might have originated in soapstone, mixed detrital clays (or wood-derived potash) are a more likely source of this component.[36]
The names assigned to fields on each diagram reflect the relative importance of key components. They do not necessarily correspond to the most abundant components in these wares, but rather are chosen to reflect the most distinctive ones. In this regard the proposed classification scheme supplements and embellishes rather than merely replaces the traditional groupings proposed by Eccles and Rackham.[37] Although comprising six compositional planes, the scheme might not adequately embrace all soft-paste American and British porcelains as new analytical data become available. Thus, by necessity, this classification scheme should be considered to be a work in progress.
No consideration has been given here to the classification of hard-paste porcelain and stone china. Both are enriched in silica and alumina compared with soft-paste porcelains. The SiO2-3Al2O3-5CaO diagram can be used to show the range of bulk SiO2/Al2O3 ratios in both types of ware. Their low lime content ensures that they plot in the aluminous silicic field shown in figure 7. Although this is an apt description of their composition, they are nonetheless quite different types of ware. Stone china—from Lund’s Bristol factory as described in Richard Pococke’s personal correspondence (1750)—is known only from the archaeological record.[38] It is a tan-colored ware with a lead-rich glaze.
Compositionally it can be distinguished from true porcelain by its titanium content (~1% TiO2), which likely originated in the use of ball clay rather than kaolinite. Indeed, this type of aluminous-silicic ware commonly contains grains of titanium oxide minerals (TiO2 polymorphs).[39] Other impurities are suggested by the composition of the melt phase. For example, although a stone china–like ware from the Brownlow Hill factory in Liverpool contains a variably aluminous melt (~15-33% Al2O3) whose composition appears to be controlled by minute mullite crystallites, some spot analyses of the matrix phase indicate an enrichment in plagioclase components (CaO, Na2O) even though the paste contains only small concentrations of lime and soda.[40] This suggests that these parts of the melt contain partly resorbed detrital grains (i.e., impurities) of plagioclase that were present in the ball clay (or silica sand) used to make this ware. Despite differences in their clay sources and hence minor element compositions, both types of silicious-aluminous wares are highly vitrified and so have a vitreous luster on broken surfaces.
Apart from their variable silica and alumina content, there is little to pick and choose between different types of true porcelain, although some eighteenth-century Russian (St. Petersburg) hard-paste wares are mildly phosphatic.[41] Compared with soft-paste porcelains, however, there are few published data for hard-paste wares, including stone china. As more data become available, it may well be that compositional variations in these groupings of wares may inspire others to propose a composition-based classification scheme for them. A word of caution is nonetheless warranted. The ability to distinguish between soft-paste and hard-paste porcelain is one of the first challenges faced by students of fine ceramics. However, in the case of early British wares, some porcelaneous objects with aluminous-silicic compositions are not readily assigned to either category. Hard-paste (“true”) porcelains manufactured by the traditional Chinese method were derived from pastes containing china stone and china clay, and, prior to the application of any subsequent overglaze decoration, were subjected to a single high-temperature firing that fused the alkali glaze to the body. In contrast, soft-paste wares were high-fired in the biscuit kiln and then low-fired in the glost kiln after being coated with a lead-rich glaze. Some British true porcelains, however, have lead-rich glazes and so were manufactured using soft-paste methods.
Consider the coffee can shown in figure 10. This object is translucent when backlit. It has an aluminous-silicic composition (see fig. 2) and a lead-rich glaze (27% PbO). On this basis, it resembles hard-paste porcelain manufacture using soft-paste methods. However, unlike typical examples of these wares (e.g., from Coalport), this coffee can has a porous, poorly vitrified body (fig. 11).[42] Since hard-paste porcelain by definition is manufactured by firing pastes containing china stone and china clay—ingredients wholly destroyed by kiln firing—this artifact is better described as an underfired, aluminous-silicic porcelain object. Its low TiO2 content points to the use of a relatively pure kaolinitic clay (rather than ball clay) in this ware, but whether the alkalis in it originated in china stone remains an open question. It probably corresponds to what has been referred to as “hybrid hard-paste” porcelain.[43] This type of ware was made circa 1790–1810 by those who emulated true porcelains but wished to avoid paying royalties to patent holders of this technology.[44] To that end, they added additional ingredients to the basic kaolinite + china stone mixture and modified the firing technique. Some such as New Hall were fired at temperatures significantly lower than Chinese-type true porcelain.[45] The underfired coffee can shown here probably dates to the early nineteenth century, but it is not know where it was made. The point being made here, however, goes far beyond simply constraining the provenance of this object, but rather draws attention to the fact that the traditional subdivision of porcelains into hard-paste and soft-paste wares is not as readily achieved as traditionally believed. The reader can judge for him/herself whether the coffee can described here should be considered hard- or soft-paste porcelain. Certainly, visual examination of this ware would lead many observers to conclude the latter, an interpretation challenged by the composition of this object. It is worth emphasizing that firing conditions should not be a criterion for classifying paste types, nor should inferred paste ingredients be used in this regard.
Some students of early ceramics will balk at the idea of having to acquire and use analytical data to classify porcelain at all. They should remember that compositional analyses of one form or another (e.g., either qualitative or quantitative) have always been required to categorize and better understand these wares. Why else would Eccles and Rackham have analyzed British porcelain more than eighty years ago? Many enthusiasts have attempted to circumvent this approach by making use of a black (UV) light. The results of this type of examination, however, can mislead as often as inform.[46]
The main objections to adopting the type of systematic, composition-based classification schemes for porcelain proposed here are likely to be based first on a general lack of familiarity with analytical data, and second on a reluctance to microsample intact porcelain objects. The first objection is readily overcome. Indeed, standard texts on British porcelain that include clear and concise sections on compositional data for these wares have been available for more than fifty years, and have been updated by more recent review articles.[47] The second objection is more serious, given the commercial value of important early porcelain objects in pristine condition. Only a small flake of the body of a porcelain object is required to undertake a quantitative analysis by modern microbeam techniques. This can be removed unobtrusively from the footrim of many utilitarian wares. In this regard collectors of eighteenth-century porcelain would do well to remember that it has long been common practice to have Tang-dynasty tomb figures authenticated by dosimetric methods (e.g., thermoluminescence), which involves drilling a small core from the figures’ base. Porcelain can be similarly microsampled without causing undue aesthetic or financial harm to the specimen. It is up to the curator or collector to decide whether the information that can be provided from the analysis of the artifact warrants this action to be taken. Although perhaps not justified simply to classify an unusually valuable porcelain object, it almost certainly is if there is any uncertainty about the origin or authenticity of the item in question.
This strategy led to the recent discovery of the first-known intact specimen of Bovey Tracey porcelain, a veritable holy grail of eighteenth-century British fine ceramics. Chemical analysis of this object, a fuddling cup, showed that it is magnesium-rich and contains barium, and closely matches the composition of the experimental M-P-Pb porcelain sherds (see fig. 3) produced by Nicholas Crisp at his Devonshire potworks circa 1767–1774. Were it not for the inquisitiveness of the owner of this important piece, its true identity would still be unknown.[48]
Conclusions
Glass is classified according to its chemical composition. Thus, the prefix “soda-lime” applies to glass that is rich in sodium and calcium, regardless of the sources of both components used in the original glass batch. The classification of early soft-paste porcelains should be no different. The broad groupings of these wares as defined by Eccles and Rackham need to be reconsidered for several reasons: some presuppose particular paste ingredients; their names can be ambiguous; and recent work has recognized numerous hybrid wares with intermediate compositions. To that end, the present contribution defines new categories of wares based on the concentration of selected major and minor cation oxide components.
The application of this scheme requires that the bulk, major-element chemical analysis for the body of a porcelain object of interest be plotted on the appropriate diagram. The suitable diagram is selected on the basis of compositional criteria outlined in a flowchart (see fig. 9). This composition-based nomenclature avoids the genetic connotations inherent to the traditional classification scheme for these wares. Its widespread use should end some of the confusion and ambiguities that have arisen since the recent discovery of diverse novel and hybrid porcelains, as well as highlight the compositional similarities and dissimilarities between them.
ACKNOWLEDGMENTS
The bulk of the analytical data on which the classification scheme proposed here is based was collected using financial support provided by grants to the author from the Natural Sciences and Engineering Research Council (Canada) and the Social Sciences and Humanities Research Council (Canada). The manuscript benefited from reviews by Ross Ramsay and Maurice Hillis.
Herbert Eccles and Bernard Rackham, Analysed Specimens of English Porcelain (London: Victoria and Albert Museum, 1922).
Geoffrey A. Godden, Encyclopaedia of British Porcelain Manufacturers (London: Barrie and Jenkins, 1988), p. 17.
For data on early, experimental wares, see J. Victor Owen, “On the Earliest Products (ca. 1751–1752) of the Worcester Porcelain Manufactory: Evidence from Sherds from the Warmstry House Site, England,” Historical Archaeology 32, no. 4 (1998): 63–75. For data on early as well as later wares, including Flight and Barr porcelain, see J. Victor Owen, “The Geochemistry of Worcester Porcelain from Dr. Wall to Royal Worcester: 150 Years of Innovation,” Historical Archaeology 37, no. 4 (2003): 84–96.
J. Victor Owen, “Geochemical and Mineralogical Distinctions between Bonnin and Morris (Philadelphia, 1770–72) Porcelain and Some Contemporary British Phosphatic Wares,” Geoarchaeology 16, no. 7 (2001): 785–802. J. V. Owen and R. Ramsay, unpublished data. I. C. Freestone, L. Joyner, and R. Howard, “The Composition of Porcelain from the Isleworth Manufactory,” Transactions of the English Ceramic Circle 18, no. 2 (2003): 284–94. Owen, “On the Earliest Products . . . of the Worcester Porcelain Manufactory.”
The first patent, applied for in 1744, was granted in 1745. See W.H.R. Ramsay, A. Gabszewicz, and E. G. Ramsay, “‘Unaker’ or Cherokee Clay and Its Relationship to the ‘Bow’ Porcelain Manufactory,” Transactions of the English Ceramic Circle 17, no. 3 (2001): 474–99; W.H.R. Ramsay and A. Gabszewicz, “The Chemistry of ‘A’-Marked Porcelain and Its Relation to the Heylyn and Frye Patent of 1744,” Transactions of the English Ceramic Circle 18, no. 2 (2003): 264–83; and W.H.R. Ramsay, G. R. Hill, and E. G. Ramsay, “Re-Creation of the 1744 Heylyn and Frye Ceramic Patent Wares Using Cherokee Clay: Implications for Raw Materials, Kiln Conditions, and the Earliest English Porcelain Production,” Geoarchaeology 19, no. 7: 635–55.
Ian Freestone, “The Mineralogy and Chemistry of Early British Porcelain,” Mineralogical Society Bulletin (July 1999): 3–7.
Ramsay, Gabszewicz, and Ramsay, “‘Unaker’ or Cherokee Clay”; and Ramsay and Gabszewicz, “The Chemistry of ‘A’-Marked Porcelain.”
J. Victor Owen and Maurice Hillis, “From London to Liverpool: Evidence for a Limehouse-Reid Porcelain Connection Based on the Analysis of Sherds from the Brownlow Hill (ca. 1755–1767) Factory Site,” Geoarchaeology 18, no. 8 (2003): 851–82.
Godden, Encyclopaedia of British Porcelain Manufacturers, p. 18.
W[illiam] D[avid] John, Nantgarw Porcelain (Newport, Mon., Eng.: R. H. Johns, 1948).
P. P. Housley, “A Study of Derbyshire Raw Materials and Their Possible Relationship to the Manufacture of Porcelain at Derby,” Transactions of the English Ceramic Circle 14, no. 2 (1991): 126–43.
J. Victor Owen, “The Como-Hudson Factories (c. 1845–77): Results of Geochemical Analyses for Quebec’s First Known Glassworks,” Canadian Journal of Archaeology 25 (2001): 74–97.
W. B. Stern and Y. Gerber, “Potassium-Calcium Glass: New Data and Experiments,” Archaeometry 46, no. 1 (2004): 137–56.
Margaret Gary, Robert McAfee Jr., and Carol L. Wolf, eds., Glossary of Geology (Washington, D.C.: American Geological Institute, 1972), p. 670.
J. Victor Owen, unpublished data.
P.D.S. St. Pierre, “Constitution of Bone China: II, Reactions in Bone China Bodies,” Journal of the American Ceramic Society 38 (1955): 217–23.
I. Freestone, “The Mineralogy and Chemistry of Early British Porcelain,” Mineralogical Society Bulletin (July 1999): 3–7.
J. Victor Owen and Michelle L. Morrison, “Sagged Nantgarw Porcelain (1813–1820): Casualty of Overfiring or a Fertile Paste?” Geoarchaeology 14 (1999): 313–32.
A. Streckeisen, “To Each Plutonic Rock Its Proper Name,” Earth Science Reviews 12 (1976): 1–33.
W. J. Cross, J. P. Iddings, L. V. Pirsson, and H. S. Washington, “A Quantitative Chemicomineralogical Classification and Nomenclature of Igneous Rocks,” Journal of Geology 10 (1902): 555–690.
T. N. Irvine and W.R.A. Baragar, “A Guide to the Classification of the Common Volcanic Rocks,” Canadian Journal of Earth Sciences 8 (1971): 523–48.
M. J. Le Bas, R. W. Le Maitre, A. Steckeisen, and B. Zanettin, “A Chemical Classification of Volcanic Rocks Based on the Total Alkali-Silica Diagram,” Journal of Petrology 27 (1986): 745–50.
J. Victor Owen, Brian Adams, and Roy Stephenson, “Nicholas Crisp’s ‘Porcellien’: A Petrological Comparison of Sherds from the Vauxhall (London; c. 1751–1764) and Indeo Pottery (Bovey Tracey, Devonshire; c. 1767–1774) Factory Sites,” Geoarchaeology 15 (2000): 43–78.
Owen, “On the Earliest Products . . . of the Worcester Porcelain Manufactory.”
Owen, “The Geochemistry of Worcester Porcelain.”
J. Victor Owen and Robin Barkla, “Compositional Characteristics of 18th Century Derby Porcelains: Recipe Changes, Phase Transformations and Melt Fertility,” Journal of Archaeological Science 24 (1997): 127–40; J. Victor Owen and Terence R. Day, “Eighteenth Century Phosphatic Porcelains: Bow and Lowestoft—Further Confirmation of Their Compositional Distinction,” Transactions of the English Ceramic Circle 16, no. 3 (1998): 342–44; J. Victor Owen and John Sandon, “Petrological Characteristics of Gilbody, Pennington, and Christian (18th Century Liverpool) Porcelains and Their Distinction from Some Contemporary Phosphatic and Magnesian/Plombian British Wares,” Journal of Archaeological Science 25 (1998): 1131–47; J. Victor Owen and John Sandon, “A Rose by Any Other Name: A Geochemical Comparison of Caughley (c. 1772–1799) and Coalport (John Rose & Co.; c. 1799–1837) Porcelains, Based on Sherds from the Factory Sites,” Post-Medieval Archaeology 37, no. 1 (2003): 79–89.
Owen, “Geochemical Distinctions.”
M. S. Tite and M. Bimson, “A Technological Study of English Porcelains,” Archaeometry 33 (1991): 10.
I. Freestone, “A Technical Study of Limehouse Ware,” in Limehouse Ware Revealed, edited by David Drakard (London: English Ceramic Circle, 1993), pp. 68–73.
Owen and Hillis, “From London to Liverpool.”
I. Freestone, “A Dated Porcelain Waster from Isleworth,” Transactions of the English Ceramic Circle 17, no. 3 (2001): 325–26.
See Freestone, “The Composition of Porcelain from the Isleworth Manufactory,” p. 294, Table 5.
Owen, “The Geochemistry of Worcester Porcelain”; Owen and Hillis, “From London to Liverpool”; Freestone, “A Technical Study of Limehouse Ware.”
See J. Victor Owen, Katherine L. Irwin, Charles L. Flint, and John D. Greenough, “Trace Element Constraints on the Source of Silica Sand Used by the Boston and Sandwich Glass Co. (c. 1826–1888), Massachusetts,” Industrial Archaeology 31, no. 2 (2007): 39–56 [in press], fig. 8.
Robert S. Carmichael, ed., Practical Handbook of Physical Properties of Rocks and Minerals (Boca Raton, Fla.: CRC Press, 1982), p. 103.
See Stern and Gerber, “Potassium-Calcium Glass,” p. 140, Table 1.
Eccles and Rackham, Analysed Specimens of English Porcelain.
Franklin A. Barrett, Worcester Porcelain and Lund’s Bristol (London: Faber and Faber, 1966).
See Owen and Hillis, “From London to Liverpool,” p. 865, fig. 2b; and Owen, “On the Earliest Products . . . of the Worcester Porcelain Manufactory,” p. 66, fig. 2c.
For the matrix phase, see the data for sample BH15 in Owen and Hillis, “From London to Liverpool,” p. 863, Table II.
J. Victor Owen, unpublished data.
Owen and Sandon, “A Rose by Any Other Name.”
Maurice Hillis, personal communication with the author, 2003.
Godden, Encyclopaedia of British Porcelain Manufacturers, p. 20.
Ibid.
J. Victor Owen, “Antique Porcelain 101: A Primer on the Chemical Analysis and Interpretation of Eighteenth-Century British Wares,” Ceramics in America, edited by Robert Hunter (Hanover, N.H.: University Press of New England for the Chipstone Foundation, 2002), p. 57.
George Savage, 18th-Century English Porcelain (London: Spring Books, 1964); Owen, “Antique Porcelain 101,” pp. 39–61.
Brian Adams, A Bovey Tracey Tin-glazed Fuddling Cup (Budleigh Salterton, Eng.: B&T Thorn and Son, 2005).
