
Door panels from a shrank made for Emanuel and Mary Herr, Lancaster County, Pennsylvania, 1768, Walnut with tulip poplar; sulfur. (Courtesy, Winterthur Museum.) The design and execution of the panels show the hand of a highly component craftsman: the inletting is crisp and even, with no knife overruns or visible tool marks. While most of the cartouche is knife-cut, tight curves were set in with specific curved gouges, confirmed by the repeated radii throughout. Most feathers on the birds are in a thumbnail style with two gouge cuts used to form the chip; the smaller feathers on the head of the large bird are punched with a triangular tool. A tracing from the right-hand door cartouche overlaid on the left indicates that the designs were transferred from a full master or half-master template for the major areas of the design. Insignificant variations between the quadrants of the cartouches are accounted for by shifting the pattern during transfer or by variations in cutting the channel.

Schrank frieze fragment, possibly Pennsylvania, 1750–1799. Walnut; sulfur. (Courtesy, Alan Andersen.) The sulfur inlay, unobscured by later finishes, highlights the color shift in period sulfur inlay from yellow when new, to white when aged. The black specks visible in the white field occur as finish and grime collect in pits formed when water vapor rising out of the wood is captured in the solidifying sulfur. These inclusions are a good indicator that the inlay is sulfur and not paste.

Cover of Pennsylvania Folklife (Fall 1977) featuring the Georg Huber schrank. Monroe Fabian’s article, “Sulfur Inlay in Pennsylvania German Furniture,” in this issue was the most accurate source of information on sulfur inlay at the time, utilizing analyses carried out at the Smithsonian Institution.

Valuables chest, Lancaster County, Pennsylvania, 1773. Walnut with tulip poplar; sulfur and white composite repairs. H. 7 1/4", W. 14 3/4", D. 8 5/8". (Courtesy, Winterthur Museum.) The central design for this small box was generated with a compass and straightedge, witnessed by the pivot points. The flower designs, slightly varying because of inletting, are from a template. The yellow tint on the top is due to a thick resin coating and stands in contrast to the chalk-white color on the tulips, which have a sparse and likely original coating.

Splint matches, origin and age undetermined. White cedar; sulfur. L. 4". (Courtesy, Winterthur Museum.) A riven white cedar splint dipped in molten sulfur makes a nonstrikable match that carries a flame once ignited.

Corner cupboard, possibly Pennsylvania, 1780–1810. Walnut with sulfur inlay and poured pewter decoration. H. 89 1/2", W. 50 1/2", D. 40 1/2". (Courtesy, Skinner, Inc.)

Detail of the decoration on the corner cupboard illustrated in fig. 6. The star pattern was defined with a straight chisel, showing out-of-line cuts that disrupt the straight ray with a jag. This indicates that the pewter was poured rather than cut from sheet goods. Pewter fills voids just as sulfur does, but it must be filed to level the soft metal.

Tombstone, St. Luke’s Lutheran Cemetery, Carroll County, Maryland, 1793. Slate; sulfur. (Photo, Brenda Hornsby Heindl.) Many of the tombstones are sculpted in baroque profile designs with inlaid sulfur highlights. Germanic designs found on sulfur-inlaid furniture are common.

Detail of a tombstone from St. Luke’s Lutheran Cemetery, Carroll County, Maryland, 179. Slate; sulfur. (Photo, Brenda Hornsby Heindl.) The deeply cut pinwheel design has sloping sides on the interior. It appears that the molten sulfur fill was intended to stop below the surface of the stone, thereby highlighting the finely crafted edge.
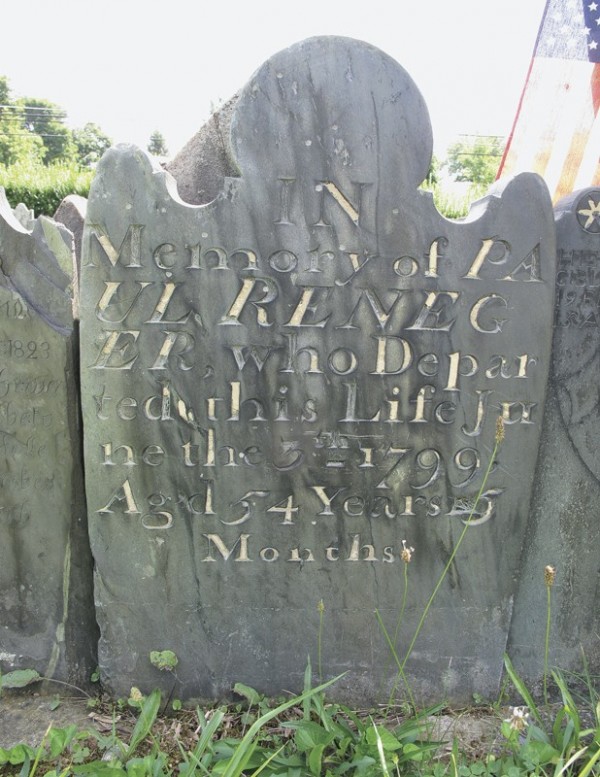
Tombstone from St. Mary’s Union Cemeteries, Carroll County, Maryland, 1799. Slate; sulfur. (Photo, Brenda Hornsby Heindl.) German or English inscriptions are found on all of the tombstones in this group.

Tombstone detail from St. Mary’s Union Cemeteries, Carroll County, Maryland. Slate; sulfur. (Photo, Brenda Hornsby Heindl.) Sulfur poured into stone tends to level without bubbles.
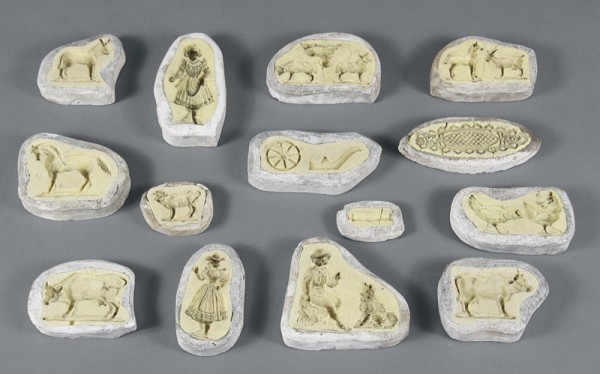
Confection molds, date and origin unknown. Sulfur; plaster. (Courtesy, Mercer Museum.) These molds, which were acquired from the Thomas Mills Company, established in Philadelphia in 1866, are probably European. The brittle sulfur cannot stand up to repeated use or environmental extremes without chipping and cracking.

One of the molds illustrated in fig. 12. This mold illustrates crossover between the carver and the mold maker. Curved gouge cuts define portions of the design.

One of the molds illustrated in fig. 12. Animal motifs are common on confection molds and decoration on American sulfur-inlaid furniture.

Butter or cookie mold, possibly Pennsylvania, 1813. Conifer. Diam. 4 1/2". (Courtesy, Winterthur Museum.) This reverse-carved mold uses patterns and a letter style common to sulfur-inlaid designs.

Router plane, European or American, date unknown. Lignum vitae; iron. L. 2 1/2". (Courtesy, Winterthur Museum.) Small router planes allowed rapid clearing of waste between the incised borders of larger designs intended to receive molten sulfur.

Procedures used in the production of a scaled down, replica panel with sulfur inlay. (Courtesy, Winterthur Museum.) The pattern is transferred to the wood using a printed image with carbonized paper. Sooting the reverse of a drawing in period work would produce a similar outline when traced. Pricked patterns using pounce powder could also transfer a design. The initial outline inletting is done freehand with a knife, while two separate curved gouges cut the thumbnail feather motif. A single gouge with an elongated, tapered cutting edge is rolled to incise slightly varying curves.

Detail of the chest façade illustrated in fig. 29. Inletting for this design was made using a knife, possibly in conjunction with a V-shaped gouge. The bottom channel is smooth from the sharp cutting edge. A knife and a straight chisel were likely used for other areas and waste removal.

Detail of the Germanic numeral “1” on the chest façade illustrated in fig. 29, showing the knife and gouge work. The chip of walnut embedded in the molten sulfur is probably waste that strayed into the molten sulfur pour. This can occur when pared-off hardened sulfur is remelted.

Detail of the Germanic numeral “8” on the chest façade illustrated in fig. 29, showing knife and chisel work. The aged sulfur is white and does not have bubble pitting. The sulfur may have been near its melting point—only slightly above the boiling point of water—so less water vapor was driven out.

Detail of fig. 17, showing the gouge made "thumbnail" cuts and straight knife cutting.

Overall of fig. 21, showing the knife and gouge carving.

Detail of a lower door panel of a schrank made for Emanuel and Mary Herr, Lancaster County, Pennsylvania, 1768. Walnut with tulip poplar; sulfur. (Courtesy, Winterthur Museum.) The knotwork pattern is compass-generated. A single or double cutter on the end of the compass beam could have described the outline before waste removal.

Detail of figure 17 during the sulfur inlay process. Solidified sulfur must be pared away with an edge tool or scraper. The hardened sulfur is abrasive and quickly dulls the tools. The area of lighter sulfur is due to overpouring. The trail, fully hardened, but still crystallizing, has a deeper color. Small details like the feathers can be “dotted in” with liquid sulfur.

The completed design illustrated in fig. 17, poured and scraped smooth. This image shows the replica panel (with a shellac finish) after cycling in an artificial aging chamber at the Winterthur Museum.

Molten sulfur poured from a ceramic crucible is difficult to control and often overpours.

A slow charcoal fire melts the sulfur with stirring. Careful melting at the lowest possible temperature is important as the sulfur begins to become more viscous above 300 degrees. Away from the heat the sulfur hardens quickly and must be poured without delay.

Detail of the chest façade illustrated in fig. 29.

Chest façade, probably southeastern, Pennsylvania, 1784. Walnut; sulfur (yellow cave-sulfur crystals from Mexico). (Courtesy, Winterthur Museum.) The sulfur crystals are formed as a result of bacterial action on organic material leached through the soil and crystallized on limestone from a cave roof. They show the natural bright yellow sulfur color in contrast to the aged sulfur inlay.
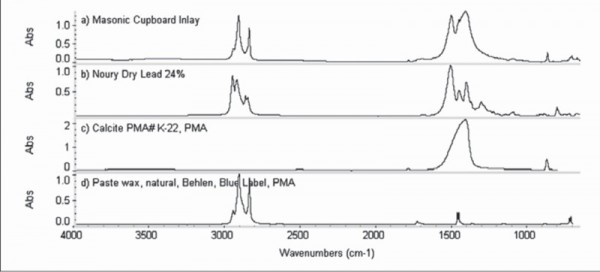
FTIR results for white inlay on the corner cupboard illustrated in fig. 31.

Corner cupboard, Bertie County, North Carolina, ca. 1795. Walnut with cypress; white lead, chalk and linseed oil filler. H. 103 1/4", W. 50", D. 20 1/2". (Courtesy, Winterthur Museum.) The inlay material for the tympanum cupboard was thought to be sulfur, but analysis indicates that it is white lead putty.

Detail of the putty inlay on the corner cupboard illustrated in fig. 31.

Detail of the inlay on a tall case clock, Reading, Pennsylvania, 1801. Birch with pine; white and colored gesso type inlay. H. 95 1/2", W. 21 3/4", D. 11". (Courtesy, Mabel Brady Garvan Collection, Yale University Art Gallery.) The composite inlay on this case has components similar to gilder’s gesso. Previously published as being wax, the inlay is unusual for its multicolor rendering.
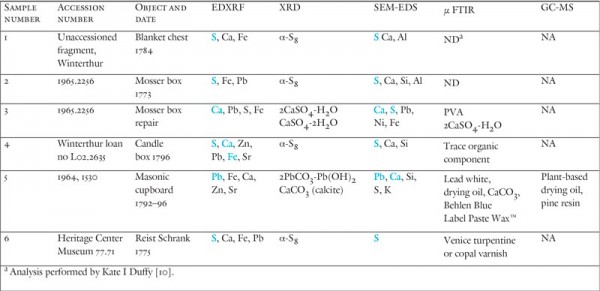
Instrumental analysis data for eighteenth-century inlays and repairs. Major elements found by EDXRF and SEM-EDS listed in blue. ND indicates no organic compounds were detected. NA indicates not analyzed.
XRD diffraction patterns used to identify smaller crystal domains found in aged sulfur inlay.

Close-up of the replica panel illustrated in fig. 25, showing spalling and color shift as a result of artificial aging.

Close-ups of the replica panel illustrated in fig. 25, showing spalling and color shift as a result of artificial aging.
The technique of sulfur inlay—most commonly observed on black walnut furniture and likely used as a rapid and attractive alternative to wood inlay—has long been considered a rare type of decoration associated with objects whose production centered on present-day Lancaster, Pennsylvania (fig. 1). However, recent research has identified more than 125 objects with sulfur-inlaid decoration and the names and locations of many original owners. It is now clear that sulfur-inlaid furniture was made in multiple shops throughout southeastern Pennsylvania as well as in Maryland, Virginia, and North Carolina. A schrank dated 1763 is the earliest documented example and a chest dated 1844 the latest object known to these authors.
Sulfur inlay is most often found on chests, schranks, and clocks, although tables, cradles, cupboards, desks, chests of drawers, straightedges, small boxes, a wall pocket, and a looking glass with this enhancement also survive. Most of these pieces are dated, many have initials identifying the owners, and a few have full names. A fragmentary frieze inlaid “Fronica” and “17” is unique in having come, presumably, from a schrank that bore a woman’s name alone (fig. 2). Typically schranks have either a man’s name or the names of a married couple. Scholars have traditionally assumed that schranks with male and female names were made for their owners’ wedding, but the dates on those objects suggest otherwise. Genealogical research does indicate that most of the people who commissioned schranks were the children of immigrants, primarily from Germany and Switzerland. This article will review previous scholarship on furniture with sulfur inlay, discuss historic uses and sources of sulfur and its chemical properties, and describe the techniques used to create sulfur and related inlays.
Historiography of Sulfur-Inlaid Furniture
In the November 1958 issue of Antiques, Frances Lichten described the decoration on the Huber family schrank illustrated as the article’s frontispiece: “the intricate filigree of ornament, executed in an obscure technique known as wax inlay . . . seems to have no early European antecedents, and only a few insignificant nineteenth century descendants on very minor artifacts” (see fig. 79 in Lisa Minardi’s article in this volume). Then, in 1960, she described the decoration as Wachseinlagen, suggesting that the origin of wax inlay was Germanic. We now know the inlay is sulfur, not wax, but Lichten’s error in identifying the ornament on the schrank is understandable because unvarnished aged sulfur inlay is an ivory-white color. Lichten’s assertion led other scholars to conclude that the material was wax paste with lead white pigment [2Pb(CO3)Pb(OH)2] intended to simulate “inlay produced in ivory, holly, and rare imported woods.” Monroe Fabian’s 1977 Pennsylvania Folklife article, “Sulfur Inlay in Pennsylvania German Furniture,” was the first publication to identify the technique correctly (fig. 3). Among the objects illustrated were a 1768 schrank that descended in the Herr family, a 1772 hanging cupboard, a 1773 box from the Mosser family (fig. 4), the 1779 Huber schrank, and a 1783 walnut chest. In 1982 Beatrice Garvan and Charles Hummel attributed the Huber schrank to Christian Huber and Peter Holl III of Manheim and Warwick townships, Lancaster County. They also speculated that Holl, who was both a joiner and a pump maker, would have been familiar with sulfur as a repair material. Mark Anderson’s 1995 article on sulfur inlay generated new interest in the subject, and in 2003 he and Jennifer Mass began intensive scientific analysis of the materials used for sulfur inlay and other allied techniques.[1]
Historic Uses and Sources of Sulfur
No direct European antecedents for American sulfur-inlaid furniture are known, but some of the designs on examples from southeastern Pennsylvania are similar to those on South German and Swiss painted furniture forms, which are typically executed with a white pigment and often have foliate motifs, initials or names, and dates. Similarly, no period documents or bills pertaining to the use of sulfur inlay have been identified either in this country or abroad. A yellow hardened paste inlay in Italian Renaissance furniture resembles sulfur inlay, but scientific analysis identifies that material as primarily orpiment (arsenic trisulfide, As2S3).[2]
The use of sulfur as a component in decorative arts and trade practice is ancient. The Egyptians are credited with developing niello, a black metallic alloy of sulfur mixed with copper, silver, or lead. Typically used as an inlay for metal, niello spread throughout Europe during the late Iron Age and is common in medieval jewelry and as an inlay on arms and armor. During the Renaissance, collectors of coins and medals used molten sulfur as an alternative to plaster for making detailed molds from originals. The process is described in Il libro dell’ arte (The Book of Art or The Craftsman’s Handbook), first published in the fifteenth century. Molten sulfur was used to cast three-dimensional forms including castings for determining the bore diameter of gun barrels, a process known as “slugging.” Household uses for sulfur included fumigation and bleaching (when burned), and for matches (fig. 5).[3]
Sulfur or sulfur and lampblack mixtures were compounded to create acid-resistant coatings and lutes (adhesives). Molten sulfur mortars were also used to join cast-iron bell and spigot pipe components as an alternative to the more common molten lead joint. Parallel use of molten sulfur and molten lead alloy is significant since the latter also occurs as a poured fill material in southeastern Pennsylvania furniture.
Most of the sulfur used in the colonies and the young republic came from vast deposits in Campania and Sicily. Europeans typically processed sulfur ore in mud-brick structures that were built on slopes around large stacked chunks of the ore and fired with lit sulfur dust. As the ore melted, it ran downhill from the base of the kiln, where it cooled and could be collected. Sulfur melts at 239 degrees Fahrenheit (115 Celsius), cools quickly, and forms a virtual cast of the surface it occupies. These very qualities made sulfur ideal for inlaying wood and stone objects in the eighteenth and nineteenth centuries.[4]
Sulfur was produced in the colonies by 1776, when the Continental Congress requested “all persons . . . in this or any neighboring Colony” to provide speedy intelligence” to the Committee of Sulfur Ore and solicited proposals “from any person or persons that are willing to engage in procuring [sulfur] . . . for the public use.” One of the first respondents was Thomas Bedwell, who lived near Philadelphia. The notes of the Committee of Safety indicate that he had access to crude sulfur and was able to process it with public assistance. Several sources in the records also refer to sulfur deposits on the Susquehanna River near York, Pennsylvania. All of this sulfur was intended for the production of gunpowder, but the same sources could have been exploited for other uses including inlay. Newspaper advertisements from southeastern Pennsylvania document the availability of sulfur through merchants and apothecaries.[5]
In addition to being a naturally occurring element, sulfur was a byproduct of merchant blast furnaces. Pyrite (iron disulfide, FeS2), which has a high sulfur content and decomposes into FeS + S at 1022°F, was used for iron production in southeastern Pennsylvania. After visiting a Pennsylvania furnace in 1772, a “Mister Goddard” recommended the addition of a limestone shelf in the upper half of the structure, “the use of which is to absorb and take to itself the crude sulfur from the metal before it falls into the hearth.” Since many other reaction pathways could occur during smelting (for example, 2 FeS2 + 11/2 O2 —> Fe2O3 + 4 SO2, 3 FeS2 + 8 O2 —> Fe3O4 + 6 SO2, FeS2 + 3 O2 —> FeSO4 + SO2), additional documentary research will be required to determine if elemental sulfur was collected and sold as a by-product of iron production in Pennsylvania. Sulfur was, however, obtained from pyrite by the Chinese as early as the third century.
Avenues for Technological Transfer
Craftsmen who worked with pewter inlay may have been familiar with the properties of molten sulfur, which has applications in different types of metalworking and gunsmithing. It is worth noting the tradition of inlaid longrifles, tomahawks, and related accoutrements that in some cases feature poured pewter inlay though no arms to date with sulfur inlay are known. An unusual corner cupboard is decorated with both poured pewter and sulfur inlay (figs. 6, 7), and other Pennsylvania objects with only pewter inlay survive. The pewter-inlaid examples are similar in form to those with sulfur and often feature names or initials and dates in the same contexts.
Masonry was an avenue for technological transfer, since molten sulfur fillers were commonly used to set iron hand-rail supports or iron cleats into large stones. The 1858 Oswego, New York, Customs’ House was erected with internal iron cramps anchored in poured sulfur, and in other applications molten sulfur socketed lightning rods and the hubs of grinding stones. Further links between stonemasonry and furniture joinery have been established by such scholars as Bradford L. Rauschenberg, who identified a large group of Davidson County, North Carolina, tombstones with carved details matching those on local case pieces. If furniture designs influenced the decoration on tombstones, there is no reason to exclude masonry practices as a source for the technique of sulfur inlay in furniture.[6]
There are numerous surviving tombstones with sulfur inlay in Maryland. Three cemeteries account for the majority of the stones: St. Benjamin’s Lutheran Cemetery, St. Luke’s Lutheran Cemetery, and St. Mary’s Union (Reformed and Lutheran) Cemeteries, all in Carroll County, Maryland. The tombstones are made of black slate, a common stone from the region, and most have epitaphs in German with decorative carving or inlay pattern. Several of the tombstones exhibit designs that mimic those found on sulfur-inlaid furniture (figs. 8-11).
As Garvan and Hummel suggested, the use of sulfur inlay on furniture may represent crossover between the pump-making and cabinetmaking trades. Iron waste pipes were joined with sulfur mortars during the nineteenth century and possibly much earlier, as the following citation indicates: “a long abandoned ancient cast iron main unearthed in the Carolinas . . . the joints poured with sulfur . . . the line laid shortly after the War of the Revolution.” If iron fittings for wooden hand reciprocated pumps were joined with sulfur lutes or pure molten sulfur pours, then Christian Huber and Peter Holl III may have been familiar with the use of molten sulfur. Although no exhaustive study of early wooden pumps has been carried out, it is possible that some had metal fittings seated in sulfur or sulfur-based materials.[7]
A seemingly unconnected but very plausible origin for the sulfur inlay technique may be the confectioner’s trade. A recently discovered cache of plaster-reinforced sulfur molds for pressing marzipan or barley sugar sweets suggests a link between wood carving and pouring molten sulfur (figs. 12-14). Patterns for sulfur molds were sculpted in pliable materials like wax or clay, but more durable ones were carved from wood, as the brittle sulfur molds could deteriorate with repeated use and require recasting from a hard pattern. Wooden molds for cookies and butter are well known and illustrate the craft of incised carved decoration quite similar to designs found on sulfur-inlaid furniture (fig. 15). Carvers who produced reverse-image wooden molds could easily carve raised relief patterns for sulfur molds. The date and provenance for the sulfur confection molds illustrated in figures 12–14. are unknown, but their motifs suggest Germanic or Swiss origin. There is no reason to believe that sulfur confection molds were not produced in the Germanic regions of Pennsylvania and that the crafts of wood-carver, furniture maker, confectioner, and confection mold maker might not have commingled. Instructions for making a sulfur mold are found in the 1820 compendium The Italian Confectioner: “oil lightly the articles you intend to mould with a hair pencil; put some sulphur in a glazed earthen pipkin . . . and melt on the fire, when it is melted and clear . . . pour it into your mould . . . the sulphur will find its level softly without blowing.”[8]
Similar processes were described in plasterer’s handbooks. According to William Millar’s Plastering—Plain and Decorative:
The moulds are generally made in sulphur, but some large manufacturers keep metal and box-wood and pear-wood moulds for stock designs. . . . Box-wood and pear-wood moulds require to be carved which only a skilled carver can do, as they have to be cut or sunk in the reverse way to give the given design. Sulphur moulds are made by dissolving the sulphur (which is generally sold in sticks) over a slow fire in an iron pot, continually stirring to prevent burning. When melted it is allowed to stand until sufficiently cool to pour on the original. . . . A good guide to know when it is fit for pouring on is to let it stand until a thin skin has formed on the top then break the skin on the side intended for pouring. . . . Should the sulphur catch fire, remove the pot from the fire and cover it with an old plaster or cement bag to extinguish the flames. It only requires attention to prevent this mishap. Burnt sulphur does not run so freely, and does not take so fine an impression as when pure.
The crafts of sulfur mold making and carving also overlapped in the production of composition ornaments. On May 25, 1805, carver John Doggett charged James Evans $2 for “2 sulphur molds for composition.”[9]
The Technique of Sulfur Inlay
Surviving examples of sulfur-inlaid furniture reveal different methods of inletting designs. Some makers formed their channels with a fixed cutter, such as a router plane (fig. 16), whereas others used a knife or chisel (for removing waste) (fig. 17). The excavated channel did not have to be perfectly rectilinear, as with wood inlay, thus a rough bottom or sloping side was perfectly acceptable for the molten sulfur pour (figs. 18-21). The outlines for floral and animal motifs were typically established with a knife. A simple V cut was common for freehand inletting, as the sulfur adheres well even without the benefit of an undercut edge. Gouges with the upper corners sharpened back to create a leading edge at the tip were used for chip cuts, and compasses were used to produce geometric patterns (figs. 22, 23). Excess hardened sulfur was pared away with edge tools and scraped smooth before a finish coating was applied (figs. 24, 25).
Replication experiments suggest that makers of sulfur-inlaid furniture could achieve better results with iron rather than stoneware crucibles, which create surface tension at the lip that inhibits smooth pouring. Experimental pours with ceramic vessels were difficult to control and typically produced a large overflow of sulfur (fig. 26). Most texts that give instructions for melting sulfur recommend the use of iron ladles heated over a wood fire, and tests with comparable implements produced an even release of the sulfur (fig. 27).
An initial attempt to replicate period sulfur-inlay produced air bubble inclusions like those observed on the original objects (fig. 28). These bubbles result from water vapor rising out of the wood by action of the hot sulfur and their consequent trapping within the solidifying inlay. The final scraping off of the hardened excess sulfur deposited above the wooden surface reveals the pocked pattern created by the trapped air bubbles. Unlike the period inlays, which generally appear an ivory-white, the replicated inlay was bright yellow, the color of the most common crystalline form of sulfur (fig. 29). This finding, together with the results of analyses carried out for Fabian’s 1977 article, raises several questions about the chemical characteristics of eighteenth- and early nineteenth-century sulfur inlay.
An ivory-colored, sulfur-based inlay could also have been made by adding a minor phase or filler such as calcite (chalk, CaCO3), gypsum (a hydrated calcium sulfate, CaSO4.2H2O), or silica (SiO2) to the sulfur. In North Carolina, cabinetmakers used two basic formulas for a non-sulfur paste or putty inlay previously identified as sulfur (fig. 30). These mixtures are composed of chalk, rosin, and linseed oil, like composition ornament formulas; or chalk, white lead, and linseed oil, like traditional stopping or glazing putty. An eastern North Carolina corner cupboard utilizes the white lead putty inlay (figs. 31, 32). In contrast, a Reading, Pennsylvania, clock has white and orange paste inlays that are chalk-based with a protein glue binder, as in traditional gesso (fig. 33). Color alone is not a reliable indicator of sulfur inlay, as non-sulfur substitutes, repair materials, aged sulfur inlay itself, or translucent surface coatings and finishing materials that infuse the inlay body, all complicate visual identification.
To begin answering the questions raised during the replication phase of this study, scientists in Winterthur’s scientific research laboratories performed a number of elemental and molecular analyses of the inlays, which involved x-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), x-ray fluorescence (XRF), colorimetry, and gas chromatography-mass spectrometry (GC-MS). XRD revealed that all the samples were composed of pure alpha sulfur, the room-temperature-stable, crystalline form of sulfur. XRD, FTIR, and Raman spectroscopy produced no evidence for fillers that could account for the ivory-white color. The minor elements in the inlays identified by SEM and XRF are the commonly observed components of surface dirt (calcium, aluminum, silicon, and iron) and later varnishes (lead driers). The absence of colorless or white fillers such as sand, chalk, or gypsum suggests that there was no original intention to create an ivory-, rather than bright yellow-, colored inlay as has been frequently suggested. Furthermore, FTIR and GC-MS confirmed that no organic binder, which could have been used to create a sulfur-filled mixture, is present in any of the samples (fig. 34). Traces of plant resins and drying oils were found on top of the sulfur, but they relate to finish rather than the composition of the inlay.
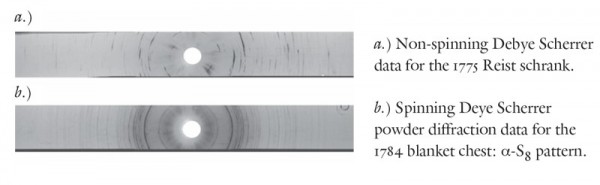
Why, then, is sulfur inlay on period objects ivory-white? A factor commonly responsible for changing the value of a mineral’s color is fluctuation in particle size. To test this theory, scientists in Winterthur’s lab performed non-spinning Debye Scherrer x-ray diffraction on an inlay sample from a 1775 schrank from the Heritage Center Museum (77.71), and a sample of the replica sulfur illustrated in illustrated figure 26. The modern inlay displayed an x-ray diffraction pattern made up of individual points or diffraction peaks, the data expected for a sample with relatively large crystallites. The same experiment on the schrank inlay revealed partial diffraction rings, consistent with the data one would obtain from a powder of finely ground particles (fig. 35). The decreased particle size observed in the schrank inlay suggests that over time the large crystals present after the molten sulfur is poured are replaced with smaller and more randomly oriented sulfur crystals.
On the macro level, sulfur crystal specimens are so temperature sensitive that they can crack from the heat of a person’s hand alone. Damage caused to sulfur crystals due to their poor heat conductivity and the resulting thermal expansion has been observed when handling sulfur crystals in natural history collections. Minerals with low thermal conductivity, like sulfur, will spall and flake off fragments, conditions that are also frequently observed on period sulfur-inlaid objects.
To further investigate this phenomenon, one of the reproduction panels was artificially aged in the labs at Winterthur. Repeated light and temperature cycling shifted the yellow color of the reproduction panel toward the ivory-white color seen in period sulfur inlays (figs. 36, 37). Additionally surface checking and minor spalling occurred as a result of the exposure cycles. The artificial aging instrument was not capable of cycling through temperatures that a piece of furniture would have experienced in an eighteenth-century household, where low to freezing temperatures, at times, would have prevailed. Low temperature cycling would further contribute to the color shift.
In summary, the mechanism that creates ivory-white sulfur inlay is repeated thermal shock over time and the repeated compressive action of the wood as it expands and contracts with humidity changes. As the inlay is subjected to these forces, the natural yellow color of the sulfur begins to appear ivory-white in color as the particle size decreases. Light exposure may play a part, but the color shift is a physical, rather than a chemical, change. Surprisingly, despite the particle size reduction, the inlay does not become powdery.
Much remains to be known about the shop-craft and aging mechanisms of sulfur inlay and the characteristics of other paste inlays, but continued research into the makers themselves may illuminate this decorative technique. Original citations in the form of bills or workshop notebooks may turn up in the future, and genealogies of cabinetmakers or allied craftsmen may shed light on sulfur-inlaid furniture. However, fifty-five years after Frances Lichten’s reference to wax inlay or Wachseinlagen, we can now identify inlays of pure alpha sulfur as well as other inlays of varying components. The origin of this short-lived decorative technique is still not known nor is the cause for its demise as a craft process. Melting sulfur can certainly be dangerous, and it gives off noxious fumes. Working with sulfur produces fine dust that workers can inhale, and workspaces and clothing become saturated with it. Perhaps craftsmen working with sulfur became annoyed by the tendency of ingested sulfur to be excreted through the skin, leading to, as one period source describes, “silvers being blackened in the pockets of those who take sulfur.”[10]
Frances Lichten, quoted in Alice Winchester’s editor’s statement, Antiques, no. 5 (November 1958): 416–7. Frances Lichten, “A Masterpiece of Pennsylvania-German Furniture,” Antiques, no. 2 (February 1960): 176–8. Monroe Fabian, “Sulfur Inlay in Pennsylvania German Furniture,” Pennsylvania Folklife 27, no. 1 (Fall 1977): 2–9. See also Monroe Fabian, The Pennsylvania-German Decorated Chest (Lancaster, Pa.: Heritage Center of Lancaster County and the Pennsylvania-German Society, 1978), pp. 38, 51. Beatrice Garvan and Charles F. Hummel, The Pennsylvania Germans: A Celebration of Their Arts, 1683–1850 (Philadelphia, Pa.: Philadelphia Museum of Art, 1982), p. 31. The cupboard, box, schrank, and chest are illustrated in Lisa Minardi’s article in this volume. Mark Anderson, Sulfur Inlay, Chester County Historical Society Antiques Show Catalogue (West Chester, Pa.: Chester County Historical Society, 1995), pp. 36–40. Jennifer L. Mass and Mark J. Anderson, “Pennsylvania German Sulfur-Inlaid Furniture: Characterization, Reproduction, and Aging Phenomena of the Inlays,” Measurement, Science, and Technology 14, no. 9 (September 2003): 1598–1607.
Bernard Demay and Christine Demay, Meubles polychromes alsaciens. (Saragossa, Editions du Bastberg: 2002), p. 33. Gislind Ritz, Alte geschnitzte Bauernmöbiel (Munich: Georg D. W. Callwey, 1974), p. 157. Heinrich Kriesel, Die Kunst des deutschen Möbels (Munich: C. H. Beck, 1970), p. 37. Siegfried Seidl, Riederbanrische Bauernmöbel (Munich, Germany: Callwey, 1973), p. 27.
Cennino d’Andrea Cennini, Il libro dell’arte, translated by Daniel V. Thompson (New York: Dover Publications, 1933), pp. 101, 130.
http://en.wikipedia.org/wiki/sulfur (accessed September 2015).
State of Pennsylvania, Minutes of the Provincial Council of Pennsylvania from the Organization to the Termination of the Proprietary Government (Philadelphia, Pa.: Jo. Severns & Co., 1852), p. 519. It is unclear where Bedwell built his operation. One congressman noted that Bedwell was in Middletown, but not whether that was Pennsylvania or New Jersey. Ibid., p. 514. Pennsylvania Evening Post, December 21, 1775. Pennsylvania Chronicle, April 13, 1772.
Martin E. Weaver, Conserving Buildings and Materials: A Manual of Techniques and Materials (New York: Wiley, 1997), pp. 64–66. Bradford L. Rauschenberg, “A Study of Baroque and Gothic-Style Gravestones in Davidson County, North Carolina,” Journal of Early Southern Decorative Arts 3, no. 2 (November 1977): 24–50
Walter Lee Sheppard, Corrosion and Chemical Resistant Masonry Materials Handbook (Norwich, Conn.: W. M. Andrews Publications, 1986), pp. 222, 226, 228.
G. A. Jarrin, The Italian Confectioner: or Complete Economy of Desserts, Containing the Elements of the Art According to the Most Modern and Approved Practice, Full and Explicit Directions Respecting Distillation, Decoration and Modeling (London: Harding, 1820), p. 120.
William Millar, Plastering—Plain and Decorative: A Practical Treatise on the Art & Craft of Plastering and Modeling . . . Together with an Account of Historical Plastering in England, Scotland and Ireland, Accompanied by Numerous Examples (London: B. T. Batsford, 1899), pp. 398-99. John Doggett daybook notation for James Evans, May 25, 1805, 64x10, Collection 330, Joseph Downs Collection of Manuscripts and Printed Ephemera, Winterthur Library.
Abraham Rees, The Cyclopedia; or Universal Dictionary of Arts, Sciences, and Literature (Philadelphia: Samuel F. Bradford and Murray; Fairman and Co., 1802-1819), 36: S. V. “sulphur” in Materia Medica entry.
