
Gew Grange soapstone quarry. (Photo, Victor Owen.)

A view of the bay at the end of the Gew Grange quarry. The pale band in the cliff is a shear zone in which soapstone formed at the expanse of the dark host rock (peridotite). (Photo, Victor Owen.)

Teapot, Lowestoft, ca. 1770. Soft-paste porcelain. H. 6". (Courtesy, Roderick Jellicoe.)

Plate, William Reid, Liverpool, ca. 1757. Soft-paste porcelain. D. 9". (Courtesy, Roderick Jellicoe.)

Coffee can, William Reid, Liverpool, ca. 1758. Soft-paste porcelain. H. 2 1/4". (Courtesy, Roderick Jellicoe.

Coffee cup, Derby, ca. 1758. Soft-paste porcelain. H. 6". (Courtesy, Roderick Jellicoe.) This enameled example has an unusual ear-shaped handle.

Vase, Derby, ca. 1760. Soft-paste porcelain. H. 6". (Courtesy, Roderick Jellicoe.)

Coffee can, Samuel Gilbody, Liverpool, ca. 1758. Soft-paste porcelain. H. 2 1/2". (Courtesy, Roderick Jellicoe.)

Mug, Samuel Gilbody, Liverpool, ca. 1758. Soft-paste porcelain. H. 3 1/2". (Courtesy, Roderick Jellicoe.)

Breakfast cup, Pennington’s, Liverpool, ca. 1785. Soft-paste porcelain. D. 4 1/2". (Courtesy, Roderick Jellicoe.)

Plate, Bow, ca. 1756. Soft-paste porcelain. D. 8". (Courtesy, Roderick Jellicoe.)

Sauceboat, Worcester, ca. 1754, Soft-paste porcelain. H. 2 3/4". (Courtesy, Victor Owen.)
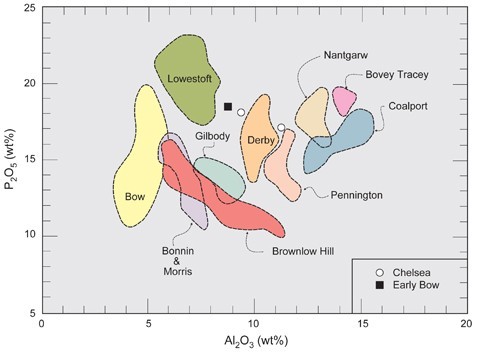
Identification of eighteenth-century porcelain factories based on bulk alumina contents (Al2O3).
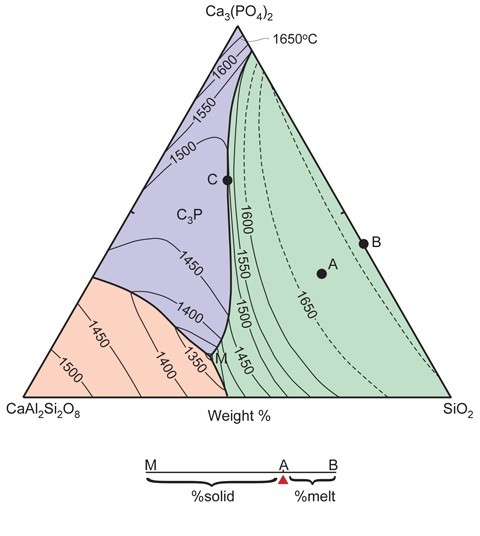
Phase diagram of how porcelain pastes melt in kiln temperatures.
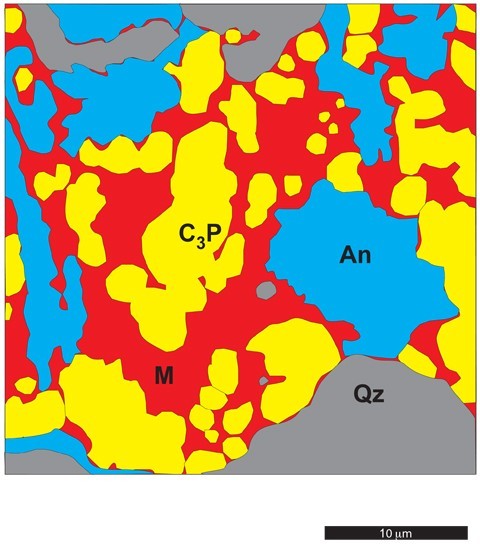
Diagram showing the shape (morpohology) during heating.

Diagram showing crystallized mineral grain.
The western world has been fascinated by Chinese porcelain since its existence was first revealed to Europeans by Marco Polo 800 years ago. Monarchs collected it, the landed gentry coveted it, and ordinary folk imitated it with inexpensive coarse earthenware. It was the ultimate status symbol. All of this ensured that it was only a matter of time before entrepreneurs—some sponsored by their regents—would seek and eventually find the secret of manufacturing their own porcelain.
In Europe, the first pseudo-porcelain was manufactured in Italy in the late sixteenth century. True porcelain was first made in Germany in the early eighteenth century, several decades before any type of porcelain was made in Britain. Early British porcelain, however, is of particular interest, not only because many different types were produced, but because large quantities of it were exported to colonial North America. As such, it can be found on many archaeological sites on this continent, even French settlements such as the Fortress of Louisbourg, in Cape Breton.[1]
The road to a domestic British porcelain industry took many turns (see table 1) because those experimenting with the manufacture of these wares only knew what the end-product should look like but had no real idea how to achieve this goal. Porcelains are white and translucent. Their translucency results from the ware partly melting (vitrifying) in the kiln. Its white color reflects the use of suitable clay and other ingredients. Identifying these ingredients was a major obstacle to those attempting to produce porcelain. As a result, their trial-and-error experiments made use of a remarkably wide range of paste mixtures. Some of these had the unfortunate property of promoting wholesale melting, and hence sagging, of the ware at peak firing temperatures. Thus, the control of kiln conditions was as much an issue as paste recipes. Fortunately for porcelain enthusiasts, all early porcelain factories created extensive waster piles in the course of their operations, and these are a fertile source of research material.
This article summarizes the character of the diverse raw materials used in porcelain pastes and glazes and the effect that these have on their chemical composition, and hence their classification. Methods by which porcelain can be analyzed are briefly discussed, after which the use of compositional fingerprints in porcelain provenance studies is considered. In order to further highlight the significance of these analytical data, a porcelain analysis is recast into a paste recipe. Using historical information concerning the amount of a key ingredient consumed by its manufacturer, recipe data are then manipulated so that an annual production rate can be estimated. Features indicative of kiln firing temperatures are also reviewed. Finally, the broad issue of collecting analytical data on archaeological ceramics is considered by citing some real-life examples.
The Raw Materials of Porcelain
Based on their experience with making coarse earthenware, early potters attempting to “reinvent” porcelain knew they had to base their experiments on mixtures of clay (for plasticity), quartz-rich sand or flint (an aplastic), and fluxing agents (to promote melting at achievable temperatures). Here consensus ended. Indeed, one of the most contentious issues for modern researchers concerns the type and source of the clay used in eighteenth-century British porcelain. It is therefore instructive to review the main types of this and other ingredients that manufacturers included in their porcelain pastes. The mineral formulae and chemical compositions of most of these ingredients are summarized in tables 2 and 3 above, respectively, and these raw materials are briefly described below.
CLAY comprises a group of hydrated aluminum silicate minerals that form minute crystals with a flaky (sheet-like) character. Clays tend to have an electrical polarity that allows positively charged ions to bond to their surface. This affects their composition.
Clays can be subdivided according to the mode of their formation, as well as their compositional and crystallographic characteristics. Primary (residual) clays form by the in situ degradation of aluminous minerals (principally feldspars) in bedrock. If transported and redeposited some distance from their source, they are considered to be secondary (sedimentary) clays. Primary clays include china clay (kaolinite) and bentonite; secondary clays include ball clays, fireclays, stoneware clays, and red marls. In terms of their composition and crystal structure, clays can be subdivided into five groups: kandites (including kaolinite), illites (e.g., illite and celadonite), smectites (e.g., montmorillonite and saponite), palygorskites (e.g., sepiolite), and vermiculite. Of these, the kandites are the most important in the preparation of porcelain pastes: kaolinite is a principal ingredient in true porcelain. Furthermore, ball clays, which are widely used in different types of ceramics and even glazes (to improve the adhesion of the glaze to the body), mostly consist of kaolinite, although detrital grains of other minerals are invariably present, giving ball clays a very wide range of compositions.[2] Early porcelain manufacturers washed these clays to remove impurities.
QUARTZ (a form of crystalline silica) in one form or another is invariably added to ceramics. To ensure the whiteness of the fired ware, it is advantageous to have a source of pure white, quartz-rich sand, although in some instances flint or chert was used instead. The presence of this mineral strongly influences the firing behavior of ceramics because it undergoes several crystallographic inversions in response to changing temperature (and/or pressure, in geological settings). Quartz gradually expands as it is heated, but there is an abrupt increase in its volume when it transforms into another silica mineral (polymorph) such as tridymite or cristobalite. These inversions are reversible, so the silica minerals contract during cooling. These crystallographic inversions can lead to cracking of the body of the ware and crazing of the glaze.
CHERT (flint) is a form of cryptocrystalline silica. It commonly occurs as nodules in limestone and chalk deposits. Rarely pure silica, it generally contains small amounts of calcium carbonate (e.g., calcite) and water. Although naturally gray, it whitens when heated (calcined), and this process also allows the chert to be easily crushed for use in ceramic pastes.
BONE ASH is made by calcining animal bones, a source of both calcium and phosphorus. Not all bones are useful in this regard—some animal bones contain impurities such as iron, rendering them less suitable for the preparation of porcelain. Cattle bones are usually used. The use of bone ash dates back to the early days of British porcelain, but it is unclear why this is the case. One possibility is that animal bones are white and readily available (and therefore inexpensive) from the knackerman. A more intriguing reason centers on the notion that adding bones to ceramic pastes would give them strength in the same manner that skeletons support vertebrate animals. Although within the realm of folklore, there is a tradition in Britain for this explanation.[3] Whatever the reason for its initial use, bone-ash is a principal ingredient in modern bone china.
Bone ash is known to influence the melting behavior of porcelain. A key issue in the investigation of historical bone-ash wares (sensu lato) is the temperature at which the bones are calcined. If “burned” at temperatures below about 1000°C, bone ash contains parts calcium oxide (CaO) for each part phosphorus oxide (P2O5) (and one part water).[4] But when added to ceramic pastes and fired at temperatures above 1000°C, the bones progressively dehydrate and lose about 25% of their calcium, which reacts with clay to form calcium-rich silicate minerals, including anorthite (calcium plagioclase). The bulk CaO/P2O5 ratio (expressed in terms of molecular proportions, see below) of eighteenth-century British phosphatic porcelains generally ranges between about 3.2 and 3.84, indicating that the bone ash at the time was high-fired, so it had already lost some of its calcium. This inference is supported by the analysis of calcined bones from the Longton Hall site, which have a CaO/P2O5 ratio of 3.3 to 3.5. Caution should be exercised in interpreting these data, however, because it is known that unglazed (biscuit) sherds from this site have had calcium leached from them, probably by circulating groundwater during 250 years of burial.[5] Bones from this site might also have been leached. Clearly, the analysis of calcined bones from other sites in which this leaching process has not occurred would be useful in better understanding the character of this ingredient and its effect on the mineralogy and composition of the wares in which it is found.
SODA ASH, a highly soluble sodium carbonate, was used as a fluxing agent in many early porcelains.
Traditionally, it was made by burning kelp.
POTASH, a highly soluble potassium carbonate, was also used as a fluxing agent. It was made by burning plants.
FLINT GLASS, or lead crystal, was used as a fluxing agent in some early porcelains (e.g., Longton Hall, Worcester; see table 1 for the dates for these and other factories). Its lead content can vary considerably. Blue flint glass (smalt) from the Caughley site proved to contain about 37 wt.% lead oxide (PbO). It also contains significant amounts of potassium. Smalt from America’s first known porcelain works, the Bonnin and Morris factory in Philadelphia, however, contains about 61 wt.% PbO.[6]
BARITE is a barium sulphate mineral. It was used in the paste of some experimental porcelains (e.g., Bovey Tracey). Substantial amounts of the element barium are also present in the glaze on some of the nineteenth-century porcelains produced at the Grainger (Worcester) works. This glaze is very low in sulphate, so unless all of the sulphur was dissipated as sulfur dioxide (SO2) during firing, the barium must originate in a source other than barite (e.g., perhaps a barian feldspar such as celsian).
STEATITE, a talc-rich rock (soapstone), was used to partly replace clay as the plastic ingredient in some early porcelains (e.g., Worcester). Steatite is the major source of magnesium in these wares. When fired, steatite can break down to yield enstatite, a magnesium-rich pyroxene-group mineral that has a low coefficient of thermal expansion. These wares therefore resisted breaking when immersed in boiling water, a definite selling point to a nation of tea drinkers. If calcite was also included in the paste, then steatite can break down to form diopside, a calcium-magnesium pyroxene group mineral. At one time, it was thought that diopside was rare in “soapstone” porcelains. Analysis of these wares from a variety of sites, however, have shown that it actually is very common, having been identified to date in all such analyzed wares except the low-calcium porcelain produced in Worcester and its Vauxhall counterpart.[7]
During the eighteenth century, soapstone porcelain manufacturers quarried their steatite from the Lizard peninsula, where this material forms narrow veins in serpentinite, a magnesium-rich rock derived from the hydration of mantle peridotite. These were small-time mining operations (fig. 1, 2), only a few tons of steatite being removed from the ground annually. It is very likely that the steatite used was adulterated by the host rock. Indeed, so-called ground “talc” recently purchased at a pottery supply store proved to have this mineral only as very a minor constituent; serpentine and other magnesium-rich minerals comprised most of the powder.
CALCITE, a calcium carbonate mineral, is the principal mineral in limestone and marble. Powdered calcite is now often referred to by potters as whiting.
DOLOMITE, a calcium-magnesium carbonate mineral, is the principal mineral in dolostone. It forms as magnesium-bearing brines circulate through and react with limestone. Thus, many limestones can be dolomitic. It may be a source of magnesium in some porcelains, although it is clear that this component generally originates in steatite.
CHINA STONE is granite that has had the feldspars altered to clay minerals by the action of hot water (hydrothermal solutions) circulating through the bedrock. It is used in true porcelain and bone china. Traditionally, British manufacturers of these wares used Cornish china stone, which tends to be extremely aluminous (i.e., is derived from peraluminous granite, which contains concentrations of aluminum in excess of that residing in feldspar group minerals in the rock).
GYPSUM is a hydrated calcium sulphate mineral. It is the likely source of small amounts of sulphur that can occur in some porcelains, especially phosphatic ones. In these wares, the sulphur seems to get trapped in phosphate minerals during firing, although some may be liberated from the paste in a gaseous state (e.g., as SO2) as the mineral breaks down. If this is the case, then paste recipes calculated from compositional data will underestimate the amount of gypsum that actually was used in the ware. In rare instances, other sulphate minerals such as barite were used by some manufacturers.
Glaze Raw Materials
Two main types of glazes were used on eighteenth-century British porcelain. Artificial porcelains were coated with lead-bearing glazes after an initial, high-temperature firing. In contrast, many true porcelains were coated with alkali-lime glazes that could withstand the very high temperature of the biscuit kiln, so only a single firing was required prior to the application of on-glaze decoration (e.g., enameling). Some true porcelains, however, have lead glazes, and so were manufactured using the traditional, two-stage soft-paste firing sequence. The lead content of the glazes on early porcelains can vary enormously (from about 10 to 55 wt.% PbO). Since typical flint glass contains in the order of 35 wt.% PbO, then clearly, at least some glazes must have made use of another source of this component (e.g., red lead or another lead oxide).
As health problems emerged in those charged with the task of dipping biscuit wares into lead glazes, alternate glazes were developed for artificial porcelains. In the 1820s, John Rose of the Coalport works won a gold medal for introducing a feldspathic glaze that contained borax as a fluxing agent. Surprisingly, analysis of the glazes on some modern British bone china shows that the use of lead has not entirely disappeared from the industry.[8]
Glaze recipes required the presence of three types of ingredients: glass forming compounds (e.g., silica, which upon solidification allows the glaze to retain the amorphous character of the molten state), stabilizers (e.g., alumina, which stiffen—increase the viscosity of—the molten glaze), and fluxes (e.g., alkalis and lead compounds to lower melting temperature). Insofar as glazes are glassy coatings on ceramic bodies, it is inherently difficult to ascertain the ingredients used in their manufacture, because virtually none (tin oxide is a rare exception) are preserved as relict grains in the glaze itself. However, compositional data suggest that early glazes were prepared from some of the same ingredients used in the paste, but in much different proportions. For example, the glazes on many soapstone porcelains can contain significant amounts of magnesium, a component generally lacking (or present only in minor concentrations) in the glazes on other types of wares.
Analytical Methods
British porcelain has been analyzed for many decades (fig. 3-11). In 1922 Eccles and Rackham published dozens of analyses of early wares that are still useful today.[9] However, over time, developments in instrumentation have allowed ever-smaller pieces of porcelain to be analyzed with greater accuracy and precision and with lower detection limits. There are many analytical tools that can be used for this purpose, but my own preference is the electron microprobe. This method has several advantages: only a small (mm-scale) piece of sample is required, and the sample does not have to be crushed. Once the sample is prepared for analysis, the microprobe can be used to determine its bulk composition, as well as the composition of individual mineral or glass grains within it. If equipped with a backscattered-electron imaging system (which generates and measures features in “compositional snapshots” of the ware), the microprobe can accurately determine the proportion of its constituent minerals. This can prove to be invaluable in studies that focus on the potential melt fertility of different types of paste, a feature that can also be addressed on more theoretical grounds using phase diagrams, as discussed below.
The microprobe uses a narrowly focused (micron-scale) electron beam to irradiate a prepared sample of porcelain (or another medium). The irradiated part of the sample emits secondary X-rays that are indicative of its composition. The sample is generally prepared for analysis by being sawn and ground flat, glued to a glass slide, and polished to a highly reflective, smooth finish. A skilled lapidary technician can successfully prepare a sample as small as 2 mm in diameter. In the case of intact objects, powder scraped from the base can be mounted in epoxy, and then polished. In this instance, however, only the composition of its constituent grains can be accurately determined, but this alone may serve to identify the ware. Sample preparation and subsequent analytical costs can vary considerably, but typically a single sherd can be analyzed for as little as fifty dollars. Furthermore, the same prepared sample (particularly if ground to a 5-times normal 150 microns thickness) can also be analyzed by other microbeam methods, such as the laser ablation microprobe, which can provide more accurate trace element data than the electron microprobe for samples containing low concentrations of these components. A more recent development is the hard X-ray microprobe, which can not only be used to measure the concentrations of trace elements, but also their oxidation state. The use of these alternate methods, however, can add substantially to analytical costs.
Categories of Early British Porcelain
There are two end member categories of early British porcelain and one intermediate variant. Artificial (soft paste) porcelains are so-named because they contain some man-made ingredients, such as fritted (melted, quenched, and ground) mixtures of various compounds. These wares are first fired in an unglazed state in the biscuit kiln (T~1250°C), and subsequently, after glazing, at lower temperature in the glost (glaze) kiln. The glaze lies thickly on the body. In these regards, these wares are fundamentally different from true (Chinese-type) porcelains, which only contain “natural” ingredients (e.g., china stone and china clay), and are given a single high temperature (T~1400°C) firing during which the glaze fuses to the body. Of course, the addition of colored decoration requires additional firing. For example, a “hardening-on” firing of the ubiquitous underglaze blue designs was introduced to minimize bleeding of the cobalt pigment into the glaze, a characteristic that plagued some manufacturers (e.g., Lund’s Bristol).
Glazes on early artificial porcelains contain lead. True porcelains usually have an alkali-lime glaze, but as already noted, some British manufacturers made use of lead glazes instead. In Britain, true porcelains were first manufactured in 1768 by William Cookworthy (Plymouth porcelain), and subsequently at Bristol. Artificial porcelain is thought to have first been made in the mid-1740s at the Pomona (Staffordshire) and Bow (London) factories (table 1).
The classification of British artificial porcelains has traditionally been based on key paste ingredients. These categories are therefore mirrored by the composition of the wares they represent. Thus, bone-ash porcelains are phosphatic, soapstone porcelains are magnesian, and frit (“glassy”) porcelains contain lead, which in most instances likely originated in the use of flint glass. The latter grouping, however, is somewhat of a misnomer, because most, if not all, artificial porcelain manufacturers fritted some of their ingredients before grinding them to a powder and mixing them with clay and water. They were very secretive about their methods, but some historical documents record their procedures, albeit often in code. William Billingsley, who during the first quarter of the nineteenth century operated a bone-ash porcelain factory in the Welsh hamlet of Nantgarw, fritted bone ash and other ingredients, ground it, mixed it with water and clay to form a fluid slurry, and then slip cast his wares.
The analysis of sherds from factory sites has revealed new types of porcelaneous wares. For example, the dominant type of ware recovered from the Limehouse works (London) is a non-phosphatic, silicious, aluminous and calcic porcelain that contains small amounts of lead. A compositionally similar ware was found among abundant bone-ash porcelain sherds at the Brownlow Hill (Liverpool) site; both have an unusual, lead-rich, tin-bearing glaze. There are, however, some notable differences between the Limehouse and Liverpool samples: those from Limehouse may contain relict bottle glass, which served as a source of calcium. In contrast, limestone was the likely source of calcium in the Liverpool samples. In addition, the tin-bearing glaze on the Brownlow Hill sherds is crystalline (i.e., it contains quartz crystals) and in this regard is perhaps unique among early British porcelains. This feature, however, likely reflects differences in the cooling history in the glost kiln rather than inherent contrasts between the character of the Limehouse and Brownlow Hill glazes. Both glazes contain particles of refractory tin oxide that would have served as nuclei on which quartz crystallites could have formed given suitable environmental conditions.[10]
There are also hybrids between the more traditional groupings of British porcelains. Most early Worcester porcelain (fig. 12) was made from pastes that contained both flint glass and soapstone; they are thus soapstone/frit porcelains. The earliest wares from this factory lacked soapstone—an ingredient previously used by the Lund’s Bristol factory, which was acquired by Worcester’s proprietors in February 1752. Prior to this, experimental Worcester pastes included flint glass and bone ash. These novel wares can be considered to be a frit/bone ash hybrid. The most important porcelain hybrid was invented in the mid-1790s by Josiah Spode, who combined ingredients of true porcelain and bone-ash porcelain pastes. In so doing, he created bone china, still an industry standard in Britain.
Compositional Signatures of British Porcelain
and Their Use in Provenance Studies
Many different factories produced phosphatic, soapstone, or frit porcelains. Some even produced more than one type of ware, as they switched recipes in response to various technical difficulties that they encountered (See table 1). In some instances, recipe changes coincided with the annexation of a competing factory (e.g., Derby abandoned frit porcelain pastes in favor of bone-ash pastes after they acquired the Chelsea works in 1770). Thus, identifying the type of porcelain simply narrows the field of factories responsible for an object’s creation. However, no two factories used identical recipes that incorporated exactly the same composition of all ingredients. Consequently, within each category of porcelain, there are compositional differences that serve to distinguish the products of particular porcelain works. These compositional signatures can be identified by analyzed bona fide wasters excavated from factory sites. Furthermore, the same analytical approach advocated here for porcelain can be applied to pottery, and preliminary results show that redware produced at various factories can have as distinctive compositional and mineralogical signatures as their more refined, porcelaneous counterparts.
Analytical data for both porcelain and pottery can be portrayed graphically as discrimination diagrams based on key components diagnostic of important ingredients (e.g., aluminium oxide [Al2O3], which reflects the amount of clay used in the paste). This approach is particularly useful for artificial porcelains, which can have a wide range of compositions within each category. Among phosphatic wares, bulk alumina contents can range from about 5 to 15 wt.% for porcelains produced at various factories (fig. 13). Distinction between true porcelains produced at different factories, however, is more equivocal, partly because few data are available for these wares, but also because they are produced from mixtures of relatively few ingredients that mostly originated from the same general area (e.g., Cornwall).[11]
Manipulating Analytical Data
Analytical data for early porcelain can be used for much more than simply identifying wares. Bulk compositional data can be recast into paste recipes, or used to predict the amount of melt that should form in the ware during kiln firing. The composition of the former melt phase in the ware provides an indication of firing conditions. These objectives will be considered in turn in the following sections.
1) Recipe Calculations
For many archaeologists, analytical data are more readily appreciated if they are expressed as proportions of paste ingredients (i.e., recipes) rather than their chemical constituents (e.g., expressed as cation oxides). Analyses of individual samples can be recast into a paste recipe provided that some information is available about which ingredients were used by the manufacturer. Historical factory records can provide detailed information about these ingredients, but the mineralogy of the samples themselves also provides important clues. In principle, recipe calculations are straightforward, but some uncertainty is introduced when the ingredients have variable compositions (e.g., sand, china stone). In contrast with earthenware, however, the ingredients used in porcelain pastes tended to be rather pure, and this both simplifies the calculation and minimizes errors associated with it.
At the heart of the recipe calculation presented here is the fact that many components in the ware originated in specific ingredients. For example, virtually all of the magnesium in soapstone porcelain has its source in steatite, whereas almost all of the phosphorus in bone-ash porcelain originates in bone ash. Since both soapstone and bone ash have predictable, well-known compositions (i.e., are stoichiometric) that vary little from sample to sample, the proportions of other elements within them are governed by the amount of magnesium and phosphorus, respectively. Thus, the proportions of these ingredients in the paste is the given by the sum of the signature element and other components in them, weighted according to stoichiometry (i.e., mineral formulae). Other types of ingredients such as rock (e.g., Cornish stone) or glass (e.g., flint glass) can be analyzed, and then treated as stoichiometric media. The only wrinkles in this type of calculation are introduced by the fact that mineral formulae are expressed as molecular proportions rather than by weight (as analytical data generally are), and some ingredients contain volatile substances (e.g., water and carbon dioxide) that are driven off during kiln firing. The first problem is readily solved by dividing through the wt.% analytical data by the molecular weight of each cation oxide (tables 3, 4). The second problem is remedied by adding back volatile components to their respective sources (e.g., water in steatite; carbon dioxide in calcite). The sample calculations in tables 4 and 5 show how an analysis of fiorcester porcelain can be converted to a plausible paste recipe. Such recipes can be calculated by other means as well (e.g., least-squares calculations, as are commonly used to calculate the modal mineralogy of crystalline rocks), but the approach advocated here has the advantage that it can be readily undertaken with a hand calculator or spreadsheet.
Calculated paste recipes are useful for comparing and contrasting the compositions of wares in a tangible, meaningful way. They can also be used to calculate annual porcelain production rates provided that the yearly consumption of a key ingredient is known for the factory in question. The only other information that is required is a reasonable average weight for typical wares from the factory. The analytical data and calculated recipes are given in weight percent. Therefore, if the amount of a key ingredient consumed annually at a given factory is known, then an annual production rate can be calculated from proportion of that ingredient in the paste. During the 1750s, approximately eighteen tons of soapstone from quarries on the Lizard peninsula were purchased annually by fiorcester’s proprietors. It is likely that nearly all of this soapstone was used in Worcester pastes, because it was expensive (about £5/ton).
Given a typical soapstone content of about 37 wt.% in Worcester pastes and assuming an average weight of 250 grams per piece of porcelain, then in the order of 180,000 objects could have been produced annually at this factory.[12] If Worcester produced more teabowls and saucers (which weigh comparatively little) than, say, dinner plates and teapots, then this value could be about half the actual production rate. Factory manifests likely provide a clear idea of the proportion of different types of wares produced at any given factory for which records have been preserved. Representative examples from the period could then be weighed, and a weighted average determined, so that a more accurate idea of this factory’s production could be calculated.
What is clear from this type of calculation is that only a few firings would have been necessary even at large factories, because kilns were stacked to roof with porcelain-laden saggars, to the extent that a large bottle kiln could easily accommodate over 20,000 objects. It is no wonder that a failed firing was financially devastating for these enterprises. It is likewise not surprising that the kiln operator was one of the most highly paid workmen at porcelain works.
2) Estimation of Kiln-firing Temperatures and Melt Fertility
Archaeologists have long been interested in reconstructing the firing conditions used in the manufacture of ceramics. In the case of coarse earthenware, this can sometimes be determined from the identity and composition of certain minerals in the ware such as iron oxides.[13] Other workers have directly measured firing temperatures associated with the burning of certain types of fuel, in everything from open fires to updraft kilns, although the application of these results to ethno-thermometric studies can be controversial.[14]
In the case of porcelain, the fact that kiln temperatures, by definition, were sufficiently high to promote partial melting of the biscuit places close constraints on firing conditions. This is because of the availability of experimental data that relate different compositions of pastes to the amount of melt that can initially form in them, and its composition at different temperatures at and above the vitrification point. The analytical data are usually presented graphically in what are referred to as phase diagrams. Phase diagrams portray the results of experiments designed to simulate natural and synthetic systems. The data are plotted either as weight percent or molecular proportions; if this information is not given explicitly, the position on phase diagrams of compounds of intermediate composition indicates which form of data is appropriate. For example, if the composition of compound “AB” plots exactly halfway between components “A” and “B” on the edge of an “A-B” (two component, or binary) or “A-B-C” (three component, or ternary) diagram, then the data should be plotted as molecular proportions. If not, weight percent data are used. The only exception to this rule occurs where A and B have the same molecular weight, a rather unlikely scenario. Both bulk and phase compositional data can be plotted on these diagrams.
The term phase refers to a physically discrete substance (solid, liquid, or gas) within the system being investigated. The diagrams can be based on one or more chemical compounds (components) that dominate the ware being studied. Binary diagrams are portrayed as simple X-Y plots, wherein the vertical axis is temperature, and the horizontal axis is composition. Ternary diagrams are triangular; each apex represents a different component. Each side of ternary diagrams represents a binary system. The vertical axis, extending perpendicular to the page, represents temperature. Thus, in essence, ternary diagrams have a thermal topography. Thermal valleys (called cotectics) extend down-temperature, and can meet at what is referred to as a thermal (ternary) minimum (point “M” in fig. 14). The position of the ternary minimum (there may be more than one) dictates the composition of the first-formed (“minimum”) melt that will form during kiln firing. The closer the bulk composition of the ceramic paste being fired is to the ternary minimum, the larger the amount of melt that will form at that temperature. Therefore, if by happenstance the paste has a minimum melt composition, then the ware will completely melt at the thermal minimum, provided that the system remains at that temperature for a sufficiently long period of time.
The example shown here (fig. 14) is pertinent to phosphatic porcelains that are sufficiently aluminous that calcium-rich plagioclase feldspar (anorthite: CaAl2Si2O8) can form. On this diagram, the bulk composition of a typical bone ash ware is represented by point A. The amount of melt that can be formed in this ware once the minimum melt temperature (point M; a temperature slightly less than 1300°C) has been reached is determined by the lever rule. To apply this rule, a line is drawn from point M, through A, to the side of the diagram (point B). The proportion of minimum melt is given by the ratio of the distances AB/AM. Of course, this system excludes alkalis, which were used as fluxing agents in these wares. Their presence lowers the minimum melt temperature and can increase the melt fertility of the paste. In this regard, phase diagrams represent only a simulation of how ceramic pastes will actually behave in the kiln.
It is important to understand the significance of the minimum melt. Regardless of the temperature of the kiln, the ware being fired will remain at the minimum melt temperature and generate a melt of fixed composition until one of the minerals in the system is completely reabsorbed. In the example used here (point “A” in fig. 14), anorthite will be the first mineral to be dissolved. Until this happens, the minerals in the paste melt in the proportions dictated by the ternary minimum. Once a phase has dissolved, however, the temperature of the ware can rise, and the composition of the melt steadily changes. It does so in a predictable way, because the melt composition tracks a thermal valley up-temperature. In the case of the phosphatic ware shown in figure 14, once anorthite has dissolved, the melt will track the cotectic, separating the phosphate and silica fields until it reaches point C, whereupon the last of the calcium phosphate will dissolve. As temperature continues to rise, the melt will follow a compositional pathway along a line connecting C and A. Silica polymorphs will continue to dissolve until the liquid has a composition corresponding to the bulk sample (point A) which will then, of course, be entirely molten.
How can these diagrams be used to reconstruct peak kiln temperatures? Temperatures on these diagrams are represented by lines of equal temperature (isotherms) that resemble contours on a topographic map. It is from these that the peak kiln temperature can be surmised. In general, however, this will be a maximum temperature, because, as already noted, the ware will often contain small amounts of a fluxing agent(s) not included in the chemical system represented by the phase diagram.
Provided that the system closely simulates the bulk composition of the ware (e.g., if it represents >90 wt.% of its bulk composition), then the composition of the melt phase (now a glass) can indicate whether or not melting was restricted to the thermal minimum. In many instances, however, the former melt phase in porcelain is adulterated by minute (submicron scale) crystallites that cannot be seen even at extreme magnification (e.g., X20,000). However, if the analyzed melt (inclusive of crystallites) compositions are plotted on the phase diagram, they can define a linear array of points between the thermal minimum and the position of the contaminating phase on the diagram. If the melt compositions instead extrapolate to a point on a thermal valley, then there should be one fewer phase present in the system (unless it started to crystallize out from the melt during cooling, and melting occurred above the thermal minimum).[15]
Isotherms indicate the temperature corresponding to this point on the cotectic. The shape (morphology) of mineral grains (crystals) helps to distinguish between relict minerals that have not been completely dissolved during heating (fig. 15) and those that have subsequently crystallized from the melt during cooling (fig. 16).[16] Thus, informed interpretation of the ware requires more than simply identifying the presence of each phase: the origin of each mineral should also be considered where observations allow.
Discussion
Connoisseurship of early British porcelain has come a long way over the past few decades. Fifty years ago, some reference books on the subject advocated distinguishing between true and artificial porcelain by scratching the base of such objects with a steel file. This would now be considered unjustified vandalism, although it is altogether a different matter if unobtrusive scrapings from the object were being taken for the purpose of microchemical analysis.
Although previous generations of researchers often relied on ultraviolet lights to help link porcelain objects to specific factories, UV lamps should not be considered a reliable tool in attribution studies because vastly different types of porcelain can fluoresce in a similar manner.[17] For example, the body of phosphatic Nantgarw porcelain and some early Worcester soapstone wares can generate very similar pinkish-brown to brick red tones when illuminated by a long-wavelength black light.[18] Furthermore, unless they are pristine, the unglazed parts of porcelain objects (e.g., the base) can fluoresce differently than a fresh broken surface. The manner in which these surfaces fluoresce can be difficult to observe because of the intense colors that can be generated by the adjacent glaze. Neither should the colors generated by the irradiated glaze be considered diagnostic, because small variations in glaze compositions can strongly influence how they respond to UV light. Furthermore, glazes on individual objects can be compositionally heterogeneous (e.g., zoned).[19] Black lights are, however, indispensable for detecting slick repairs to damaged specimens.
Modern connoisseurs tend to be more objective than some of their predecessors who flourished at a time when folklore seemed to rule the day. Commonly, the opinions of certain enthusiasts were often accepted unchallenged simply because of the august source of the information. Regrettably, some factory sites were excavated without the supervision of professional archaeologists. As a consequence, in some instances, large quantities of sherds were removed from the ground with little in the way of documentation. Indeed, many of these sherds, where not casually dispersed among other collectors, reside in dusty basements, uncataloged or even unnumbered (or sharing the same cryptic number as dozens of other sherds), and so have limited value from the point of view of serious research. Fortunately, a quick review of the recent literature shows that the times are changing. Apart from “rescue digs” where archaeologists “save” sherds from bulldozers, such excavations are now conducted in a much more systematic manner, so that the significance of individual sherds can more fully be appreciated.
There are, of course, situations in which even well-documented potsherds can mislead. These include cases where circulating groundwater has modified the mineralogy and composition of the wares, or where sherds from old factory sites actually originated elsewhere; many factories used broken dishes made elsewhere in their ceramic pastes (this material was referred to as “grog”). In the first instance, glazed sherds can preferentially preserve their original compositions better than biscuit samples.[20] In the second instance, it is best to conduct analytical work on obvious wasters, particularly sagged samples, those that are still attached to kiln furniture or firebricks, or those with aesthetic characteristics (e.g., shape or decoration) or markings that are unequivocally associated with the factory from which they have been excavated.
The application of widely used analytical methods to these wares has shed new light on the history of the porcelain industry in Britain. Many of the traditional opinions—some so entrenched that they once approached dogma—have been objectively tested. For example, it has been demonstrated that kiln-firing losses at Nantgarw were largely related to difficulties in controlling kiln temperatures rather than any inherent deficiency in the paste.[21] Based on the analytical data for these wares, belated advice can nonetheless now be offered to William Billingsley, Nantgarw’s proprietor, on how he could have rendered his paste more refractory, so that it would have generated less melt at the extreme kiln conditions at which it was often fired.[22]
It has also been shown that, despite claims to the contrary, Worcester’s early proprietors did indeed experiment with novel pastes prior to acquiring Lund’s Bristol factory.[23] This can only lead to a better appreciation of early Worcester wares and the innovative individuals who created them. Similarly, the long-held belief that America’s first domestic porcelain, produced in the early 1770s at the Bonnin and Morris factory in Philadelphia, is virtually indistinguishable from Bow has also been refuted.[24] Although Bonnin and Morris hired former Bow staff, they produced a distinct type of bone-ash porcelain. Unlike any other phosphatic ware that has been analyzed to date, this Philadelphia porcelain made use of small amounts of flint glass. This can be detected by the microprobe analysis of less than a milligram of material surgically removed from the unglazed base of any suspected Bonnin and Morris porcelain object.
Analytical data can also point to possible linkages between seemingly unrelated factories. For example, possible connections between the Limehouse and Brownlow Hill works is suggested by some of the wares (stone china; Si-Al-Ca rich porcelain) recovered from these sites. This inference is supported by the fact that a William Ball worked at both factories, although it is unclear whether or not this was one and the same individual.
For some inexplicable reason, it is largely collectors who appreciate antique porcelain; comparatively few archaeologists are interested in these wares. Perhaps this is due to the fact that the ceramic assemblages recovered from colonial archaeological sites are dominated by utilitarian wares (redware, stoneware, etc.). Despite this, subordinate quantities of porcelain artifacts are widespread at many domestic (e.g., houses), commercial (e.g., up-scale taverns), and military (e.g., officer’s quarters) sites. These fragments are usually given a generic name (e.g., “British soft-paste porcelain,” “Chinese export,” etc.), and then disregarded. And yet, what better evidence is there for high-end social status than these lustrous bits of dishes lying beneath the ground? And how easily they can be traced to a specific factory, especially if they originate in Britain, data for porcelain made elsewhere (except perhaps oriental wares) being comparatively sparse at present.
The analysis of ceramic sherds from defunct factory sites has provided a comprehensive compositional fingerprint for many of the major British porcelain manufactories, and with a few exceptions, sherds from colonial sites can be convincingly linked to specific potworks. For example, Bow (London) and Christian/Pennington (Liverpool) porcelain has been identified at the Fortress of Louisbourg.[25] A fragment of the same type of Liverpool porcelain has also been recovered from the Bonnin and Morris factory site. The significance of the latter sherd is unclear. Either someone discarded their favorite teacup after it had been damaged, or perhaps this object was used as a template for designing some Bonnin and Morris porcelain. This is one question, however, that cannot be answered in the lab.
Conclusion
The microchemical analysis of all types of archaeological ceramics has revolutionized the interpretation of these wares. No longer is ephemeral connoisseurship required to identify pottery and porcelain artifacts. Instead, a more egalitarian situation has arisen whereby with a small budget the archaeologist can characterize the nature of even tiny fragments of undecorated wares that would otherwise confound even the cognoscenti in this field. Comparing the analytical results with an ever-expanding database compiled from sherds from factory sites, the origin of these samples can often be convincingly determined.
The data can be used for much more than provenance studies. Armed with a rudimentary understanding of phase diagrams, many of which were constructed specifically for this purpose, details about technical difficulties faced by the manufacturers of early porcelain can be assessed in a quantitative manner.
Predictions about melt fertility can be compared with measured amounts of the glass phase in the porcelain, and the composition of this phase places important constraints on maximum kiln temperatures. This type of information is crucially important to appreciating how manufacturers responded to the challenges presented by the nascent porcelain industry in Britain.
A wider understanding of these methods of analysis and the interpretation of the analytical data can readily be gained by undergraduate archaeology and ceramics students who elect to enroll in introductory physical chemistry, mineralogy, and igneous petrology courses. It is to be hoped that these types of courses will soon be routinely included in archaeological and ceramics arts curricula.
ACKNOWLEDGMENTS
Rob Hunter is thanked for providing the opportunity to present this review article in Ceramics in America. It is largely based on earlier work that was supported by Natural Sciences and Engineering Research Council, and Social Sciences and Humanities Research Council of Canada operating grants to the author. Thanks are also extended to the many museum curators and ceramics enthusiasts who generously made available sherds for chemical analysis, and to Rod Jellicoe for making available photographs of the porcelain objects.
J. Victor Owen, “Provenience of Eighteenth-Century British Porcelain Sherds from Sites 3B and 4E, Fortress of Louisbourg, Nova Scotia: Constraints from Mineralogy, Bulk Paste and Glaze Compositions,” Historical Archaeology 35, no. 2 (2001): 108–21.
F. Hamer, and J. Hamer, The Potter’s Dictionary of Materials and Methods, 4th ed. (London: A & C Black, 1997).
Harry Frost, personal communication, 1998.
Victor Owen, “Compositional and Mineralogical Distinctions Between Bonnin and Morris (Philadelphia, 1770–1772) Phosphatic Porcelain and Its Contemporary British Counterparts,” Geoarchaeology (in press).
J. Victor Owen, and T. E. Day, “Assessing and Correcting the Effects of the Chemical Weathering of Potsherds: A Case Study Using Soft-Paste Porcelain Wasters from the Longton Hall (Staffordshire) Factory Site,” Geoarchaeology 13 (1998): 265–86.
J. Victor Owen, B. Adams, and R. Stephenson, “Nicholas Crisp’s ‘porcellien’: A Petrological Comparison of Sherds from the Vauxhall (London; c. 1751–1764) and Indeo Pottery (Bovey Tracey, Devonshire; c. 1767–1774) Factory Sites,” Geoarchaeology 15 (2000): 43–78. Owen, “Provenience of Eighteenth-Century British Porcelain Sherds from Sites 3B and 4E, Fortress of Louisbourg, Nova Scotia.”
M. Bimson, “The Composition of Vauxhall Porcelain,” Transactions of the English Ceramic Circle 13, no. 3 (1989): 226–27. Owen, Adams, and Stephenson, “Nicholas Crisp’s ‘porcellien’: A Petrological Comparison of Sherds.”
J.Victor Owen, “Geochemistry of Phosphatic- and Silicious/Aluminous-Porcelain Sherds from the Coalport Factory Site,” in D. Barker and W. Horton, Post-Medieval Archeology 33 (1999): 3–93, appendix, 82–87.
H. Eccles, and B. Rackham, Analysed Specimens of English Porcelain (London: Victoria and Albert Museum, 1922).
I. Freestone, “A Technical Study of Limehouse Ware” in D. Drakard, ed., Limehouse Ware Revealed (Beckenham, Kent: English Ceramic Circle, 1993), pp. 68–77. J. Victor Owen, “A Preliminary Assessment of the Geochemistry of Porcelain Sherds from the Limehouse Factory Site, London” in K. Tyler, and R. Stephenson, The Limehouse Porcelain Manufactory, MoLAS Monograph 6 (2000), pp. 61–63.
J.Victor Owen and P. Williams, “Compositional Constraints on the Provenance of a True-Porcelain Chocolate Mug from the Rockingham Inn (c. 1796–1833) Site, Bedford, Nova Scotia,” Canadian Journal of Archaeology 23 (1999): 51–62.
J.Victor Owen and J. Sandon, “Petrological Characteristics of Gilbody, Pennington, and Christian/Pennington (18th-century Liverpool) Porcelains and Their Distinction from Some Contemporary Phosphatic and Magnesian/Plombian British Wares,” Journal of Archaeological Science 25 (1998): 1131–47.
R. Philpotts and N. Wilson, “Application of Petrofabric and Phase Equilibria Analysis to the Study of a Potsherd,” Journal of Archeological Science 21 (1994): 607–18.
O. P. Gosselain, “BonWre of the Enquiries. Pottery Firing Temperatures in Archaeology: What For?” Journal of Archeological Science 19 (1992): 243–59.
J.Victor Owen and M. L. Morrison, “Sagged Nantgarw Porcelain (1813–1820): Casualty of OverWring or a Fertile Paste?” Geoarchaeology 14 (1999): 313–32.
Ibid.
George Savage, 18th-Century English Porcelain (London: Spring Books, 1964), pp. 56–62.
See Owen and Morrison, “Sagged Nantgarw Porcelain (1813–1820).”
J.Victor Owen and R. Barkla, “Compositional Characteristics of 18th-Century Derby Porcelains: Recipe Changes, Phase Transformations and Melt Fertility,” Journal of Archaeological Science 24 (1997): 127–40.
J.Victor Owen and T. E. Day, “Assessing and Correcting the Effects of the Chemical Weathering of Potsherds: A Case Study Using Soft-paste Porcelain Wasters from the Longton Hall (Staffordshire) Factory Site,” Geoarchaeology 13 (1998): 265–86.
Owen and Morrison, “Sagged Nantgraw Porcelain (1813–1820).”
J.Victor Owen, J. O. Wilstead, R. Williams, and T. E. Day, “A Tale of Two Cities: Compositional Characteristics of Some Nantgarw and Swansea Porcelains and Their Implications for Kiln Wastage,” Journal of Archeological Science 25 (1998): 359–75.
J.Victor Owen, “On the Earliest Products (c. 1751–52) of the Worcester Porcelain Manufactory: Evidence from Sherds from the Warmstry House Site, England,” Historical Archaeology 32 (1998): 60–72.
J.Victor Owen, “Compositional and Mineralogical Distinctions between Bonnin and Morris (Philadelphia, 1770–1772) Phosphatic Porcelain.”
Owen, “Provenience of Eighteenth-Century British Porcelain Sherds from Sites 3B and 4E, Fortress of Louisbourg, Nova Scotia.”
